- Pakistan notifies revised control lists under export control act RADIO PAKISTAN
- Pakistan updates export control lists to align with global non-proliferation rules Arab News
- Trends – National Control Lists of items Business Recorder
- Govt revises…
Author: admin
-
Pakistan notifies revised control lists under export control act – RADIO PAKISTAN
-

Pharrell calls for empathy from US leaders after being honored at Black Music Collective event
LOS ANGELES — At a pre-Grammy event celebrating Black music’s cultural power, Pharrell Williams used the Recording Academy stage to deliver a prayer — not for himself, but for U.S. leaders.
“I want to pray for the leaders of this nation and…
Continue Reading
-

Stock market today: Live updates
Traders work on the floor of the New York Stock Exchange (NYSE) in New York, US, on Wednesday, Jan. 28, 2026.
Michael Nagle | Bloomberg | Getty Images
Stock futures fell Friday, a day after the S&P 500 posted a second consecutive losing session. The moves followed sharp declines in gold and silver, as the rallies that took the precious metals to record highs lost momentum.
Futures tied to the broad market index were down 0.8%, and Nasdaq 100 futures lost about 0.9%. Dow futures dropped 355 points, or 0.7%.
Gold futures dropped more than 4%, while contracts tied to silver plunged 12%. Despite the declines, gold and silver remain higher over the past year by 80% and 209%, respectively.
Wall Street also turned its eyes to Washington after President Donald Trump said Thursday night that he would announce his choice to replace Federal Reserve Chair Jerome Powell on Friday morning. Former Fed Governor Kevin Warsh has emerged as a favorite in prediction markets, but BlackRock’s fixed income chief Rick Rieder and National Economic Council director Kevin Hassett have also been in contention.
Apple shares inched lower even after the company beat fiscal first-quarter earnings and revenue expectations, aided by a significant surge in iPhone sales. Data storage stock Sandisk popped 19% on the back of strong guidance. KLA Corp lost more than 8% after guidance for non-GAAP gross margin in the fiscal third quarter came in light.
“This week brought the first wave of major tech earnings, with investors focused on results, guidance, and AI spending as a key market driver. … A clear theme is emerging, in our view,” said Angelo Kourkafas, senior global investment strategist at Edward Jones.
“Companies are ramping up AI related infrastructure spending, and markets are rewarding those that can turn these investments into earnings,” he added. “Firms without a clear monetization strategy are facing more scrutiny.”
He added that while the tech sector is still expected to deliver strong profit growth, it’s slowing from earlier quarters as other sectors accelerate. This development supports “what we see as this year’s key theme: a broadening of market leadership,” Kourkafas said.
Week to date, the S&P 500 and Nasdaq have each added nearly 0.8%. The 30-stock Dow is down nearly 0.1% on the week.
Continue Reading
-
Syrian government, Kurdish forces announce integration deal – Dawn
- Syrian government, Kurdish forces announce integration deal Dawn
- Kurdish-led SDF agrees integration with Syrian government forces Al Jazeera
- One Syria, reclaimed: How Syrians read the victory against YPG TRT World
- Saudi Arabia supports agreement…
Continue Reading
-
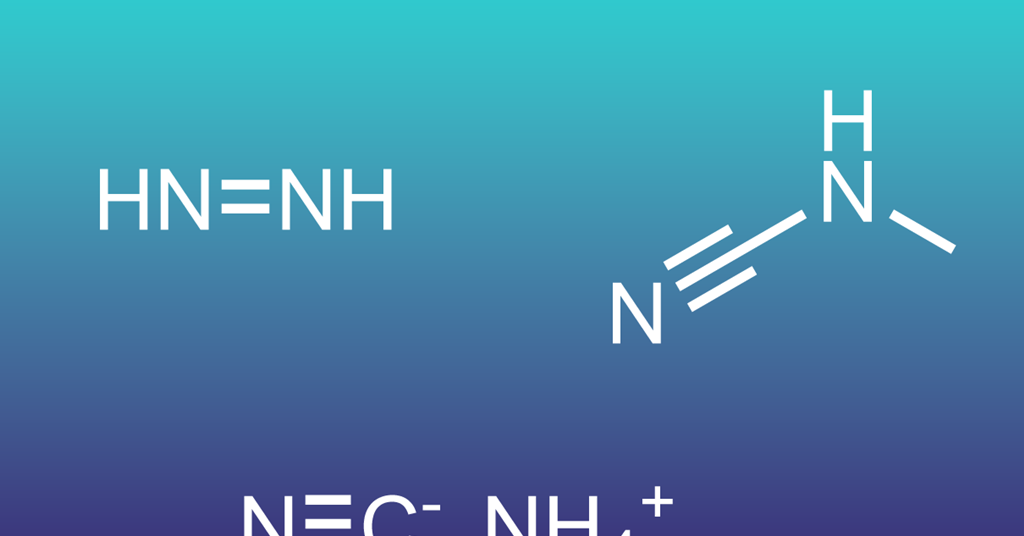
Three nitrile molecules identified as fresh targets for life-hunting astronomers | Research
Scientists have uncovered three nitriles in lab-made interstellar ice that could serve as new targets for astronomers searching for prebiotic chemistry in the depths of space.
Astrochemists have been interested in nitrile compounds for many years…
Continue Reading
-

FUJIFILM GFX ETERNA 55 Firmware Version 1.04 Released – Display Delay of External Monitor Improved
FUJIFILM has released firmware version 1.04 for the GFX ETERNA 55, addressing external monitor display latency and critical stability issues. This update reduces display delay by approximately 40ms when using SDI or HDMI outputs under specific…
Continue Reading
-

How to have the best Sunday in L.A., according to Ty Dolla Sign
For Ty Dolla Sign, the perfect Sunday begins in the sky, traveling back to Los Angeles from wherever his career has last taken him. The singer, producer and multi-instrumentalist lives in constant motion — our interview had a few…
Continue Reading
-
Bisan Owda says TikTok restores account but limits visibility – Daily Times
- Bisan Owda says TikTok restores account but limits visibility Daily Times
- Gaza-based journalist Bisan Owda regains TikTok account after outcry Al Jazeera
- CAIR in the News, January 30, 2026 CAIR
- 🇺🇸 Adam Presser, the newly appointed CEO of…
Continue Reading
-

The best books to read in 2026
Though it’s still early 2026, the turbulence of the last few weeks is overshadowing its novelty. We are finding refuge in books, both to make sense of the bad news vortex and to welcome escape. It’s a big year for books. Gov. Gavin Newsom…
Continue Reading
-

Women’s Champions Cup 2026™ Coaching Legacy Programme launched
The FA, FIFA and WSL Football announce Women’s Champions Cup 2026™ Coaching Legacy Programme to power the next generation of elite female coaches.Against the backdrop of the FIFA Women’s Champions Cup 2026™, The…Continue Reading