By Christine Ji
The company is boosting the storage capacity of its devices, a move thought to be a prelude to expected Siri enhancements later this year
Apple announced its new iPhone 17e and iPad Air, and is expected to…

By Christine Ji
The company is boosting the storage capacity of its devices, a move thought to be a prelude to expected Siri enhancements later this year
Apple announced its new iPhone 17e and iPad Air, and is expected to…

The latest advisory includes Egypt, Israel, Saudi Arabia and Qatar, according to a State Department official.
Published On 3 Mar 2026

The 33-year-old is out-of-contract after playing three seasons with Melbourne Renegades
Melbourne Renegades coach Cameron White has conceded Adam Zampa is unlikely to…

Stay informed with free updates
Simply sign up to the Oil & Gas industry myFT Digest — delivered directly to your inbox.
US liquefied natural gas exporters are racing to capitalise on a 50 per cent price surge in European and Asian markets triggered by the conflict in Iran, which knocked out supplies from LNG powerhouse Qatar.
Venture Global and Cheniere Energy, two of the largest US producers, are seeking to squeeze additional LNG volumes from facilities in Texas and Louisiana and bring more capacity online as consumers from the UK to Japan brace for supply shortages.
Traders and other buyers of US LNG — once described by the Trump administration as “freedom molecules” — are also rerouting their cargoes to take advantage of skyrocketing prices as customers battle for supplies.
“With the largest available incremental LNG capacity in the world the United States will play a critical role during this historic disruption in the market. Venture Global stands ready to help keep the markets stabilised and supplied,” Mike Sabel, chief executive of Venture Global, told investors on Monday.
Analysts have warned that the loss of Qatari LNG could trigger a severe new energy crisis, just four years after the loss of Russian gas supplies spiked European prices and hammered the continent’s economy until American gas began arriving.
Venture Global shares closed up almost 20 per cent on Monday while Cheniere closed up 5.6 per cent, as investors bet the two giant LNG exporters would benefit from the surge in spot prices.
The Center for LNG, an industry lobby group, said US providers contracted on a “free-on-board” basis, which meant traders were able to redirect US cargos after purchase to provide much greater flexibility in a crisis.
“US LNG destination flexibility allows exporters and their customers to redirect cargoes when geopolitical tensions arise,” said executive director Charlie Riedl. “That said, no single supplier can immediately substitute for another at scale.”
The crisis was sparked when an Iranian drone attack forced QatarEnergy’s Ras Laffan LNG plant to shut down. It produces about a fifth of global supplies. The company has not provided an update on damage to the facility or when it can resume production.
LNG from Qatar and the UAE also flows to global markets through the Strait of Hormuz, which Tehran moved to shut in response to the US-Israeli war on Iran.
Natural gas prices in Europe settled 39 per cent higher at €44.51 a megawatt-hour, which is the highest in about a year. UK natural gas prices settled up 45 per cent at 113.79 pence a therm.
In contrast, US natural gas prices settled up just 3.5 per cent at $2.96 per million British thermal units.
The US surpassed Qatar and Australia as the world’s largest LNG exporter in 2023 and last year shipped more than 100mn metric tonnes overseas. Several new plants are under construction but will not begin operating for months or years.
Golden Pass, a massive new facility on the Gulf coast in Texas, backed by ExxonMobil and QatarEnergy, should begin producing LNG within weeks. But it will take months to reach full capacity.
Analysts said US producers would be unable to compensate for a long shortfall in Middle Eastern supplies.
“Nothing can make up for the loss of Qatari LNG,” said Saul Kavonic, head of energy research at MST Marquee, an investment bank.
“If the shutdown is prolonged, or worse the LNG infrastructure is damaged, it portends a larger gas market shock than in 2022 when Russia turned off pipeline gas to Europe. Gas prices could retest their record highs set in 2022.”
Scott Shelton, energy specialist at TP ICAP, an interdealer broker, said the US didn’t have enough spare capacity to contain a price surge.
“Whatever we can put on a boat we are going to send,” he said. But “even if prices go up 100 per cent from here, we are still limited.”
Venture Global sells just over 30 per cent of its LNG cargoes at spot prices, compared with less than 10 per cent for Cheniere, which helps explain the bigger rally in its share price on Monday.
But traders with free-on-board cargoes they could sell into the markets would also reap a fortune, analysts said.
“The commodity traders with US supply, the trading arms of Cheniere and Venture Global and anyone who has purchased US LNG and can trade those cargoes on international markets will benefit,” said Alex Munton, analyst at Rapidan Energy Group.
“They can now sell on those cargos at 50 per cent higher prices.”

Happy Women’s History Month! For the second year in a row, the New York Theatre Guide staff is celebrating the women in the theatre industry making waves on Broadway and beyond. They’ve shared some of their favorite female theatre artists…

At least 200 civilians have been killed since the start of the US-Israel war on Iran last weekend, according to rights groups, as people inside Iran told the Guardian they were fearful of a rising death toll.
The Iranian Red Crescent Society said…

CM Punk retained the world heavyweight championship on Saturday, defeating Finn Balor at Elimination Chamber. Punk’s victory kept the the WrestleMania 42 main event between himself and Roman Reigns alive. Now, both Reigns and…

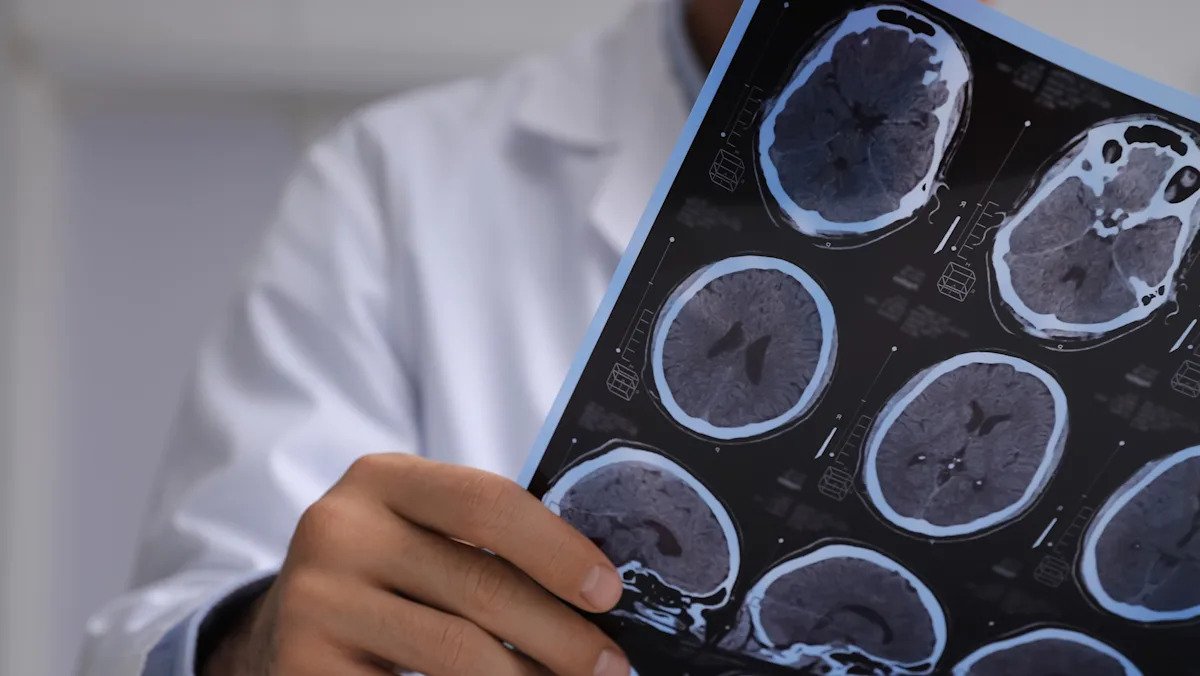
A new study has uncovered a troubling potential link between microplastics and cancerous prostate tumors. While the study doesn’t prove causation, it raises questions about the effects microplastics may have on the development of certain cancers.