AI hardware needs to become more brain-like to meet the growing energy demands of real-world applications, according to researchers.
In a study published in Frontiers in Science, scientists from Purdue University and the…

AI hardware needs to become more brain-like to meet the growing energy demands of real-world applications, according to researchers.
In a study published in Frontiers in Science, scientists from Purdue University and the…

SHENZHEN, China, Dec. 16, 2025 /PRNewswire/ — Huawei Digital Power’s Commercial and Industrial Hybrid Cooling Grid Forming Energy Storage System (C&I GFM ESS) has successfully passed a stringent extreme ignition test…

A British defence journal has revealed the serial numbers of India’s four Rafale fighter jets shot down by Pakistan in military clashes in May, exposing India’s hollow claims of air superiority.
According to an investigative report published…
Certain strains of Escherichia coli produce the natural product colibactin, which is a chemically unstable genotoxin, and increasing evidence suggests that colibactin is associated with colorectal carcinogenesis. Previous work indicted that…

An international team of researchers led by scientists from the Max Planck Harvard Research Center for the Ancient Mediterranean (Leipzig, Germany) and the University of Bologna (Italy) has reconstructed, for the first time, the genetic and…

As requested by the European Commission, EFSA focussed on the risk of infection of EU dairy cattle and poultry with the specific H5N1 genotype virus that is circulating in US dairy cattle and outlined…

Sepsis, as a major challenge in global emergency and critical care medicine, is a life-threatening organ dysfunction caused by a dysregulated host immune response to infection.1 Its incidence and mortality rates remain consistently…

In 2025, 32.7% of people aged 16-74 in the EU used generative artificial intelligence (AI) tools. Most people used them for personal purposes (25.1%), while 15.1% used them for work and 9.4% for formal education.
This information comes from data on the use of ICT in households and by individuals published by Eurostat today. The article presents a handful of findings from the more detailed Statistics Explained article on digital economy and society statistics – households and individuals.
Among EU countries, the use of generative AI tools was most widespread in Denmark (48.4%), Estonia (46.6%) and Malta (46.5%).
In contrast, the lowest shares of people using generative AI tools were recorded in Romania (17.8%), Italy (19.9%) and Bulgaria (22.5%).
Source dataset: isoc_ai_iaiu

The FSA issued a warning earlier this year that some Dubai-style chocolate products may be unsafe for people with allergies. The FSA is aware that some of these products may contain allergens such as peanut and sesame that are not declared on the label. This information has been shared with businesses and industry groups so they can take action to make sure the products they sell are safe and correctly labelled.
The FSA is currently reviewing sampling data from products on sale to check whether they meet the required food safety and labelling standards. Until the work is complete and the full results are known, the FSA is advising consumers with allergies to avoid Dubai-style chocolate as a precaution.
“Dubai-style chocolate has become hugely popular, but we’ve found that some products contain peanut and sesame that aren’t declared on the label. For someone with allergies, this could be dangerous.
With Christmas just around corner, there is a risk that some products on sale may not meet our strict UK standards.
People with an allergy should not eat Dubai-style chocolate. If you’re buying a gift for someone who lives with allergies, our advice is to avoid buying these products. This includes all allergies, not just peanut and sesame. People without allergies can consume these products, especially where they are supplied by reputable brands and retailers.
People should be able to trust that food in UK shops is safe and that it’s what it says it is, so we’re reminding businesses of their responsibility to ensure the safety of the food products they sell. We’ve also shared our concerns with allergy charities so they can help us make sure consumers have the information they need to make informed choices.
We’re continuing to monitor these products and will provide further advice, so sign up to our food alerts to stay up to date.”
Rebecca Sudworth, Director of Policy at the FSA
“CTSI fully supports the FSA’s precautionary advice and share their commitment to protecting the safety and health of consumers, particularly when it comes to allergens in food; these can be fatal to those with food hypersensitivity.
The legal requirements on this are clear – any food containing allergens needs to be clearly identified and labelled as such to allow consumers to make informed and safe choices. To not do this is illegal and also highly dangerous as it makes such foods unsafe to those with food allergies. We urge all food businesses, including retailers and importers, to take immediate steps to comply. Businesses who are not sure if they are affected by this warning should contact their local Trading Standards service for advice and guidance.
The public should be assured that Trading Standards professionals nationwide – whether advising businesses or enforcing compliance – are working closely with the FSA and affected companies to ensure products meet all safety and labelling requirements. Together, we can help keep people safe and maintain trust in the food supply chain.”
Jessica Merryfield, Chartered Trading Standards Practitioner and Head of Policy and Campaigns at the Chartered Trading Standards Institute (CTSI)
For people who do choose to buy Dubai-style chocolate, FSA advice is to buy from a reputable retailer and check that the product label is in English and contains the following information:
For more information on food allergies and how to stay safe, visit food.gov.uk.
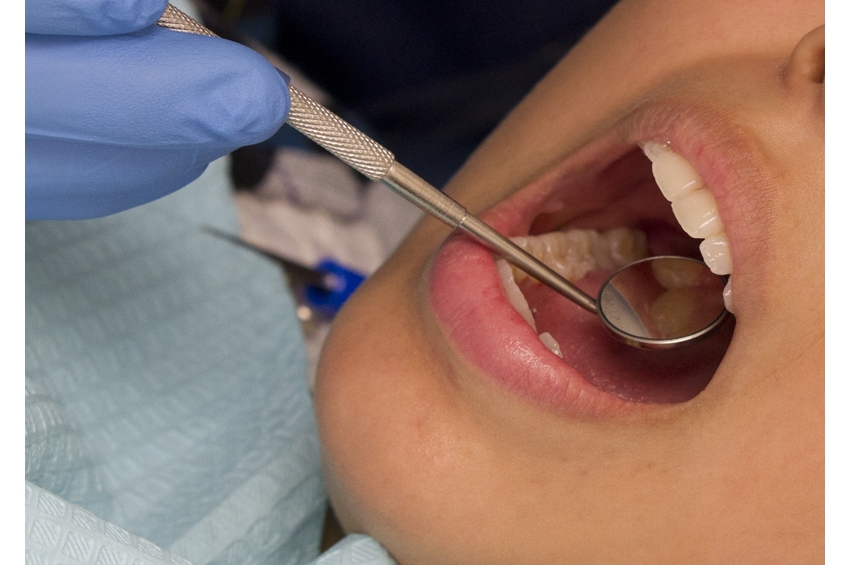
A new American Heart Association scientific statement details how oral health may affect cardiovascular outcomes and highlights how prevention and treatment of gum disease may reduce risk of…