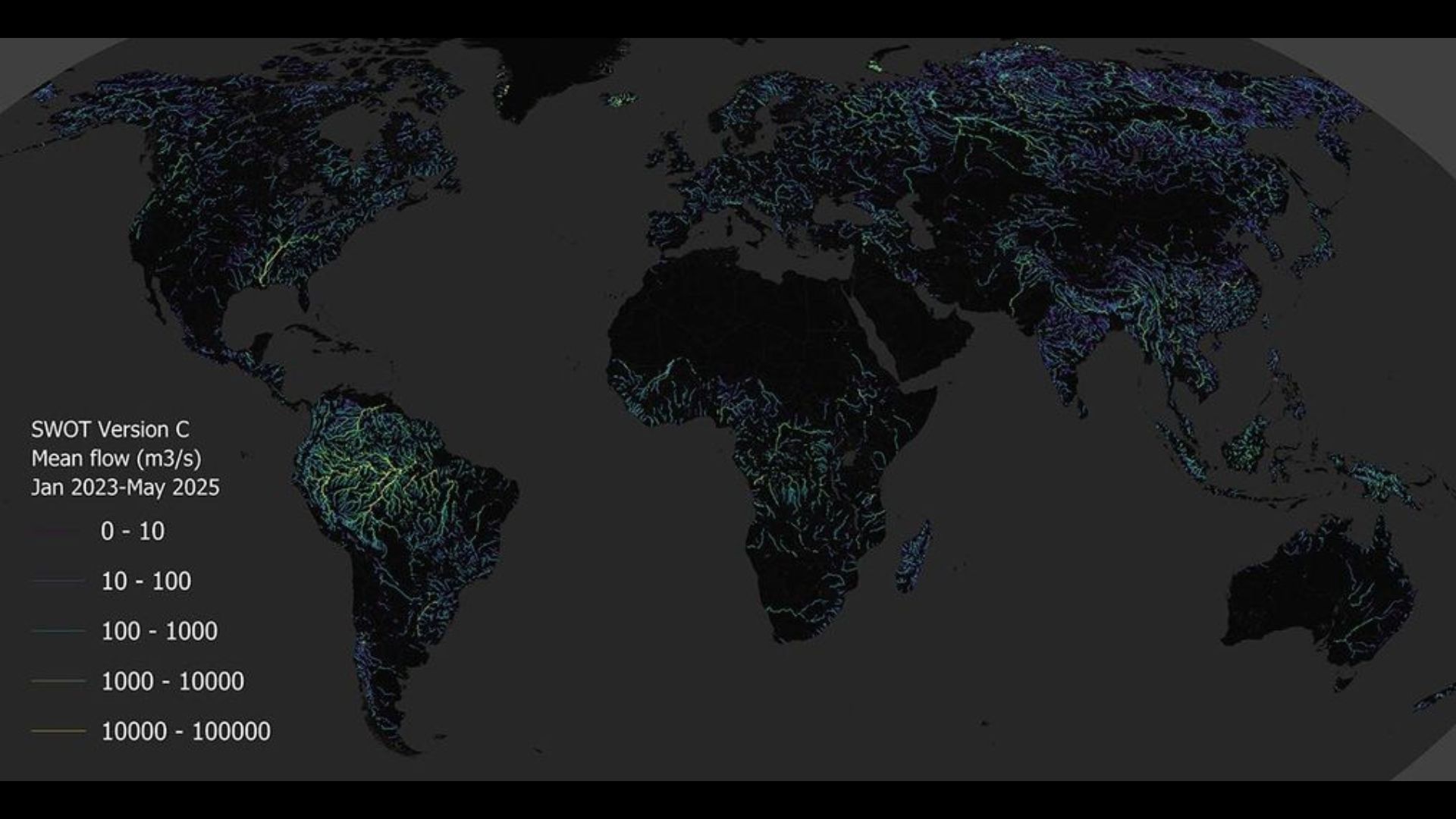
From fast-moving rivers to trickling creeks, scientists around the world work to measure discharge, or the volume of water flowing past a point per second. Discharge is the number that turns “the river is high” into “this could flood…
Author: admin
-

JSAUX Split EveryDay Case for Switch 2
Designed for daily convenience, the new series offers three modular configurations to match any lifestyle.
HONG KONG, Jan. 29, 2026 /PRNewswire/ — JSAUX, a leading gaming hardware brand, today announced the official launch…
Continue Reading
-

Kevin Durant moves to 10th all-time in 3-pointers
With his first 3-pointer on Wednesday night, Kevin Durant passed Vince Carter for 10th on the all-time 3-pointers made list.
Kevin Durant’s ascent through the NBA’s record book took another turn Wednesday night when he passed Vince Carter for…
Continue Reading
-

Dhaka–Karachi Direct Flights Resume After 14 Years
File photo of Biman Bangladesh Airlines Karachi- A special ceremony will be held on Thursday night at the Karachi airport to celebrate a direct flight between Pakistan and Bangladesh, restoring non-stop air connectivity between the two countries…
Continue Reading
-

Gohar Ejaz warns of 10M job losses for Pakistan
LAHORE: The former minister for commerce, Gohar Ejaz, has expressed concern over the trade deal between the European Union (EU) and India and warned that it could put the employment of over 10 million people in Pakistan at risk.
Speaking in…
Continue Reading
-

Ischemic stroke increases expectant mother’s risk for another stroke during or soon after pregnancy
Having had a stroke caused by blocked blood vessels (ischemic stroke) more than doubled an expectant mother’s odds of having another stroke during pregnancy and within six weeks of childbirth, according to a preliminary study to…
Continue Reading
-

Nicki Minaj’s US citizenship questioned as she flaunts Trump Gold Card visa
Rapper Nicki Minaj flaunted her Trump Gold Card visa as she declared herself to be Donald Trump’s number one fan on Wednesday.
The…
Continue Reading
-

10 million BISP beneficiaries to get payments through digital wallets from new fiscal year
– Advertisement –
KARACHI, Jan 29 (APP):National Assembly Standing Committee on Poverty Alleviation and Social Safety, while reviewing progress on conversion of BISP beneficiary accounts to digital banking channels on Thursday, was informed that…
Continue Reading
-
PIA privatization process concludes with signing of transaction documents – RADIO PAKISTAN
- PIA privatization process concludes with signing of transaction documents RADIO PAKISTAN
- PM Shehbaz, CDF Munir attend ceremony held for signing of documents regarding PIA’s privatisation Dawn
- PIA privatisation process concludes with signing of…
Continue Reading
-

AI Tool Helps Map 3D Cochlear Hair Cells
The cochlea is the spiral-shaped structure within the inner ear responsible for our sense of hearing. To fully understand hearing functions and open the door to new hearing loss treatments, scientists require intricately detailed views…
Continue Reading