A study conducted in part by Chicago’s Northwestern Medicine found that tanning beds not only triple the risk of melanoma, but can also damage DNA across nearly the whole skin surface.
Northwestern Medicine and the University of California,…

A study conducted in part by Chicago’s Northwestern Medicine found that tanning beds not only triple the risk of melanoma, but can also damage DNA across nearly the whole skin surface.
Northwestern Medicine and the University of California,…

On December 14, 2025, a terrorist attack targeted a Hanukkah celebration (“Chanukah by the Sea”) at Sydney’s Bondi Beach, organized by Chabad of Bondi. Australian authorities confirmed at least 12 deaths (including one shooter)…

More than 170 illuminated tractors and trucks have paraded through the Yorkshire Wolds.
The fourth annual charity run set out from Driffield on a 30-mile route around the surrounding villages on Saturday evening.
The event, which raises money for…

Photography by ThirDWheel Photo
MITCHELL, S.D. — No. 14 Dakota Wesleyan Women’s Basketball hosted the College of Saint Mary Flames in a GPAC conference contest.
The Tigers took control of the game early, thanks to…
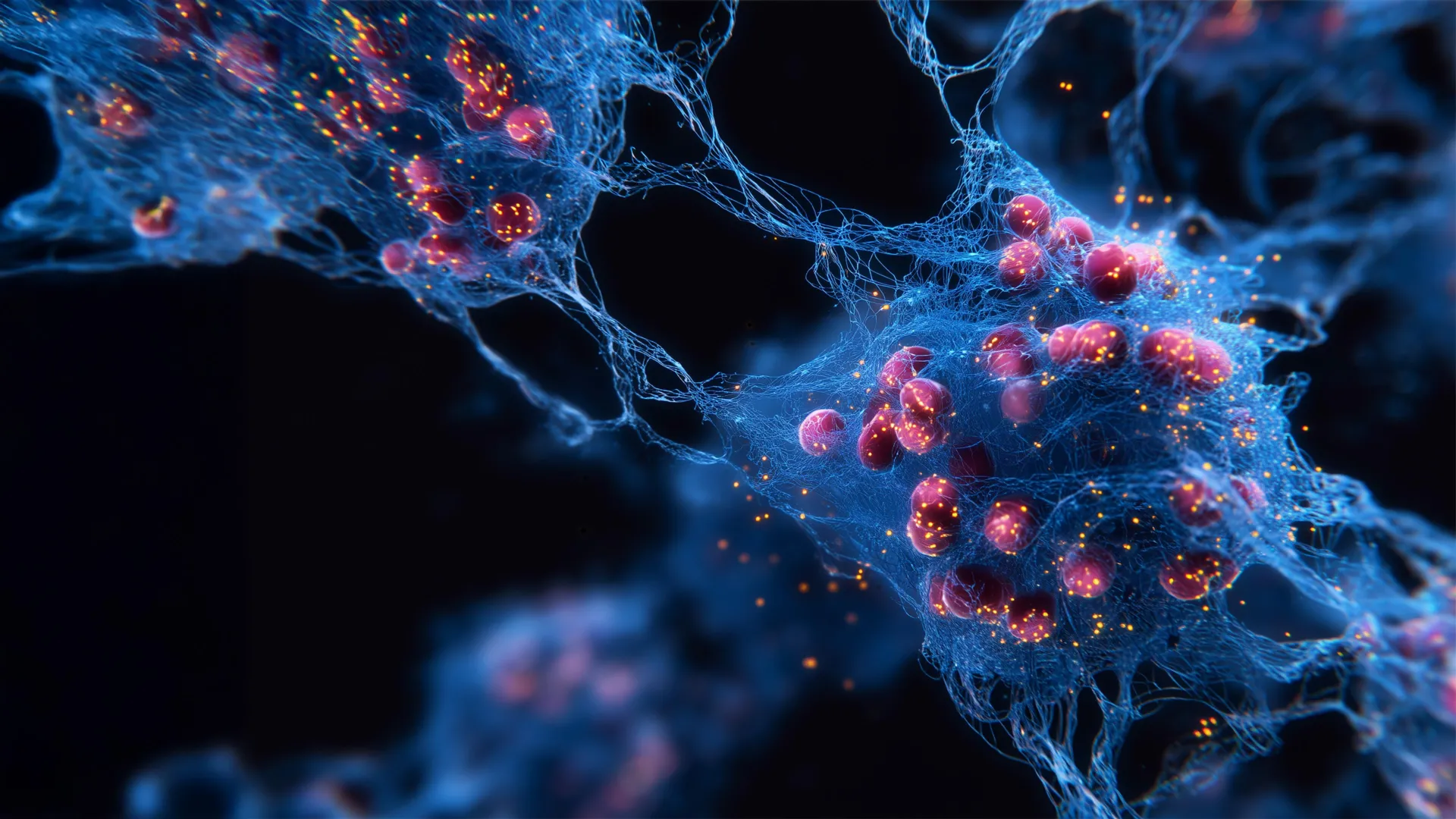
One potential strategy for treating a wide range of illnesses involves targeting senescent cells. These cells — also known as “zombie cells” — stop multiplying but fail to clear themselves from the body as healthy cells normally do. They appear…
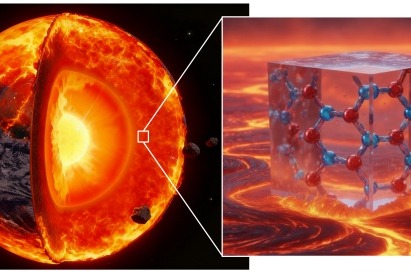
Recent studies have found the existence of vast, hitherto unknown reservoirs of primordial water thousands of kilometers below the Earth”s surface, which could offer clues to understanding the process of the planet’s…

With dozens of…