A new research paper was published in Volume 17, Issue 11 of Aging-US on November 14, 2025, titled “Methylglyoxal-induced glycation stress promotes aortic stiffening: putative mechanistic roles of oxidative stress and cellular…
Author: admin
-
Annual Changes to the Nasdaq-100 Index® – Nasdaq
- Annual Changes to the Nasdaq-100 Index® Nasdaq
- Seagate, Alnylam Are Among Six Companies Set to Join Nasdaq 100 Bloomberg.com
- Nasdaq Announces Annual Reconstitution of Nasdaq-100 Index, Adding Six New Companies Quiver Quantitative
- NDX News Today, Dec 13: Nasdaq-100 Index’s Strategic Shake-Up for 2025 Meyka
- Walmart was too late for a Nasdaq-100 spot — but these 6 stocks made the cut MSN
Continue Reading
-

Prime Video Hits Pause on Error-Filled AI Recaps
Amazon launched a limited beta of AI-generated Video Recaps for selected in-house Prime Video shows last month — titles like Fallout, Jack Ryan, The Rig, Upload and Bosch. But now the feature has made a generative AI about-face, with reports of…
Continue Reading
-

Home batteries subsidy overhauled with $7.2bn injection as Australians rush to take up discount | Renewable energy
Discounts for larger systems will be wound back under a popular home battery scheme as the program’s budget is tripled.
The federal subsidy, which has been in place for five months, will get a generous top-up to $7.2bn across four years after initially being earmarked to cost $2.3bn, the energy minister, Chris Bowen, said.
The fund was thought to be running out rapidly, in part because households were installing systems up to the maximum subsidised size to take full advantage of the one-time offer.
Bowen said he expected the $2.3bn to be depleated in the coming year.
“We’ve been installing consistently 1,000 a day batteries each and every working day and a little bit less on Saturdays, but still around 500 every Saturday and around 1,000 every working day,” Bowen said.
“I would say it was even more successful than we thought.”
Under the scheme, eligible households and small businesses have been able to secure a 30% discount on a home battery when installed alongside new or existing rooftop solar.
Sign up: AU Breaking News email
Subsidies were available on batteries with capacities of between 5kWh and 100kWh, with the rebate applied to the first 50kWh.
Bowen on Saturday confirmed the first 50kWh of a system would still be eligible for support, but discounting would not be as generous per kWh for medium- and larger-sized batteries.
Staggering support in line with battery size would encourage more households to get the “right-sized” battery, the government said, and keep the scheme open to more households.
“We know that someone who puts a battery in can reduce their bills by up to 90% and if they already have solar panels, that’s a saving of around $1,000 a year,” Bowen said.
“And if you put solar panels in a battery in at the same time, that’s a saving of around $2,000 a year.”
From 1 May, systems up to 14kWh – deemed suitable for small households – would get the full 30% discount for each kWh.
Discounting would then taper off for medium-sized kits and again for large systems above 28 kWh.
Nepean Solar chief executive, Jim Hill, said the changes were a “huge relief”.
“A boom-and-bust cycle has been a hugely impactful feature of this industry, so this sensible change that ensures the long-term viability of our sector is a huge relief and we welcome it,” the head of the Sydney-based solar and battery installer said.
“As a small business, we need to be able to plan for the ordering of stock, training and upskilling staff, and indeed the taking on of new apprentices. This approach allows us to do this with confidence.”
The Smart Energy Council chief executive, John Grimes, welcomed the funding boost and backed the rebate changes to allow more households and businesses to get access.
“We are a responsible industry that believes in spreading the benefits of solar and batteries to as many people as possible,” he said.
“If that means changes to the rebate, we support that.”
Households that add a home battery can expect $600-$900 per year in savings on energy costs on top of the bill benefits from solar, based on Australian Energy Market Commission figures.
Continue Reading
-

Hollywood Came Together at Dôen’s Holiday Fundraiser for Planned Parenthood Los Angeles
On Thursday evening, Dôen returned to Mother Wolf for its annual holiday fundraiser benefiting Planned Parenthood Los Angeles, welcoming a fashion-forward and film-heavy crowd for a gathering rooted in advocacy as much as celebration. The…
Continue Reading
-

Jello Biafra auctions his 1989 Toyota Celica
Jello Biafra, former Dead Kennedys frontman and outspoken Bay Area punk icon, is parting with a car as storied as his career: his 1989 Toyota Celica GT convertible.
After nearly three decades as his daily driver, the blue droptop is now up for a…
Continue Reading
-

Derivatives, Legislative and Regulatory Weekly Update (December 12, 2025)
Client Alert | December 12, 2025
From the Derivatives Practice Group: This week, the CFTC was especially active, issuing several no-action letters, including to designated contract markets and derivatives clearing organizations regarding event contracts and in response to a request from ISDA regarding Part 43 and Part 45 requirements.
New Developments
Acting Chairman Pham Announces Implementation of U.S. Treasury Market Reforms. On December 12, CFTC Acting Chaiman Pham announced the CFTC had approved a proposed order to grant a limited exemption necessary for the Chicago Mercantile Exchange Inc. and the Fixed Income Clearing Corporation to make their existing cross-margining arrangement available to certain customers with appropriate safeguards. [NEW]
U.S. Senate Passes Procedural Vote for Mike Selig, Paving the Way for a Final Vote in the Senate. On December 11, the Senate voted 52-47 to approve a resolution that sets up the final vote for Mike Selig’s confirmation as CFTC Chairman. [NEW]
CFTC Staff Issues No-Action Letters Regarding Event Contracts. On December 11, the CFTC’s Division of Market Oversight and Division of Clearing and Risk announced they have taken a no-action position regarding swap data reporting and recordkeeping regulations in response to requests from multiple registered entities, including designated contract markets and derivatives clearing organizations. According to the announcement, the Divisions will not recommend the CFTC initiate an enforcement action against certain registered entities or their participants for failure to comply with certain swap-related recordkeeping requirements and for failure to report to swap data repositories data associated with binary option transactions executed on or subject to the rules of the registered entities, subject to the terms of the no-action letters. [NEW]
CFTC Staff Issues No-Action Position Relating to Designated Contract Market Procedures. On December 11, the CFTC’s Division of Market Oversight announced it has issued a no-action letter to Small Exchange Inc., a designated contract market, which addresses certain procedures related to dormancy. The no-action position is time-limited and subject to the terms and conditions in the Division’s no-action letter. [NEW]
Acting Chairman Pham Announces Withdrawal of “Outdated” Digital Assets Guidance. On December 11, CFTC Acting Chairman Pham announced that the CFTC will withdraw “outdated” guidance related to actual delivery of “virtual currencies,” given the substantial developments in crypto asset markets. The CFTC said that the withdrawal of the guidance will enable the CFTC to continue its ongoing work to implement the recommendations in the President’s Working Group on Digital Asset Markets report. [NEW]
CFTC Staff Issues No-Action Letter Regarding Part 43 and Part 45 Requirements. On December 11, the CFTC’s Division of Market Oversight took a no-action position in response to a request from the International Swaps and Derivatives Association regarding certain data requirements under Part 43 and Part 45 of the CFTC’s regulations to reduce unnecessary and excessive regulatory burden and associated costs. [NEW]
Acting Chairman Pham Announces CEO Innovation Council Participants. On December 10, CFTC Acting Chairman Pham announced the first round of CEO Innovation Council participants, representing exchanges. The CFTC said that the CEO Innovation Council will engage in public discussion of market structure developments in derivatives markets and that further information on the CEO Innovation Council will be released once details are finalized. [NEW]
Acting Chairman Pham Announces Regulatory Clarity for U.S. Access to Markets. On December 9, CFTC Acting Chairman Pham announced that the CFTC’s Market Participants Division, Division of Clearing and Risk, and Division of Market Oversight issued a no-action letter to harmonize three separate definitions of “U.S. person,” among other things, under the CFTC’s Dodd-Frank Act cross-border swap framework. According to the CFTC, the letter simplified and consolidated existing no-action positions that address almost 15 years of regulatory uncertainty and promotes harmonization with SEC regulations. [NEW]
CFTC to Accelerate Publication of Backlogged COT Data. On December 9, the CFTC announced that it is accelerating the publication of Commitments of Traders reports that were interrupted during the lapse in federal appropriation. According to the CFTC, the revised timeline will eliminate the report backlog by December 29, 2025. [NEW]
Acting Chairman Pham Announces Launch of Digital Assets Pilot Program for Tokenized Collateral in Derivatives Markets. On December 8, CFTC Chairman Pham announced the launch of a digital assets pilot program for certain digital assets, including BTC, ETH, and USDC, to be used as collateral in derivatives markets; guidance on tokenized collateral; and withdrawal of outdated requirements given the enactment of the GENIUS Act. The CFTC said that the announcement follows the tokenized collateral initiative Acting Chairman Pham launched in September as a part of the CFTC’s Crypto Sprint to implement recommendations in the President’s Working Group on Digital Asset Markets report. [NEW]
Acting Chairman Pham Announces First-Ever Listed Spot Crypto Trading on U.S. Regulated Exchanges. On December 4, Chairman Caroline D. Pham announced that listed spot cryptocurrency products will begin trading for the first time in U.S. federally regulated markets on CFTC registered futures exchanges. This announcement follows recommendations by the President’s Working Group on Digital Asset Markets and stakeholder insights from the CFTC’s Crypto Sprint and cooperative engagement with the Securities and Exchange Commission. The Crypto Sprint also launched public consultations on all other recommendations from the President’s Working Group report relevant to the CFTC.
Acting Chairman Pham Announces Reforms to Wells Process, and Amendments to the Rules of Practice and the Rules Relating to Investigations. On December 1, Chairman Caroline D. Pham announced the Commission is amending its Rules of Practice and its Rules Relating to Investigations. The amended Rules of Practice seek to enhance the transparency of the Commission’s enforcement actions, including changes to ensure an accurate and complete administrative record by improving internal memoranda to the Commission when the Division of Enforcement recommends an enforcement action.
New Developments Outside the U.S.
ESMA Appoints Marie-Anne Barbat-Layani and Christopher P. Buttigieg as the New Members of its Management Board and Renews Armi Taipale’s Mandate. On December 11, ESMA appointed Marie-Anne Barbat-Layani of Autorité Des Marchés Financiers (France) and Christopher P. Buttigieg of Financial Services Authority (Malta), as the new members of its Management Board. The Board of Supervisors has reappointed Armi Taipale of Finanssivalvonta (Finland), for a second mandate. [NEW]
ESMA Chair Verena Ross to Step Down at the End of Her Current Term. On December 10, ESMA announced that its Chair, Verena Ross, has decided to not renew her term as Chair for a second mandate. According to ESMA, she will continue her work as ESMA’s Chair until the end of her contract on October 31, 2026. ESMA said that it will now launch the process for selecting a new Chair. [NEW]
ESMA Announces Supervisory Expectations for the Management Body in the Form of 12 High Level Principles. On December 10, ESMA published its Final Report on the Supervisory Expectations for the Management Body, outlining ESMA’s expectations for the management bodies of the entities under its supervision. ESMA said that the 12 high-level principles are directed at entities supervised by ESMA and those looking to obtain an ESMA license, and are designed to set out ESMA’s core expectations in the form of outcomes. [NEW]
ESMA Welcomes Commission’s Ambitious Proposal on Market Integration. On December 4, ESMA announced that it welcomes the European Commission’s legislative proposal on market integration and supervision. According to ESMA, the package represents a major step towards deeper and more efficient EU capital markets and reflects many of the recommendations set out in ESMA’s 2024 Position Paper on building more effective and attractive capital markets in the EU.
ESMA to Launch Common Supervisory Action on MiFID II Conflicts of Interest Requirements. On December 2, ESMA announced that it will launch a Common Supervisory Action (CSA) with National Competent Authorities on conflicts of interest in the distribution of financial instruments. The CSA will assess how firms comply with their obligations under MiFID II to identify, prevent, and manage conflicts of interest when offering investment products to retail clients.
New Industry-Led Developments
Global Standard-Setting Bodies Publish Assessment of Margin Requirements for Non-Centrally Cleared Derivatives. On December 12, the Basel Committee on Banking Supervision (BCBS) and the International Organization of Securities Commissions (IOSCO) published a report that reviews the implementation of margin requirements for non-centrally cleared derivatives. IOSCO said the report concluded that the framework has been effectively implemented and finds no evidence of material issues. The BCBS-IOSCO Working Group on Margining Requirements recommended ongoing monitoring through supervisory information exchange and the sharing of experiences among member authorities. [NEW]
ISDA Responds to Bank of England on Gilt Market Resilience. On December 5, ISDA responded to the Bank of England’s discussion paper on gilt market resilience. In the response, ISDA encourages the Bank of England, before introducing any significant policy changes that would affect the functioning of the gilt repo market, to consider the prudential requirements on capital and liquidity in relation to repo transactions, in conjunction with monetary policy and financial stability, to avoid unintended and detrimental consequences for the UK gilt market and firms’ risk management practices.
ISDA Publishes Note on Determining Initial Reference Index for New Trades referencing CPI-U. On November 25, ISDA published a Market Practice Note to recommend a specific methodology that market participants could elect to use for the purposes of determining the Initial Reference Index for certain new inflation derivative transactions given that the Bureau of Labor Statistics has confirmed it will not publish the October 2025 level of the “USA – Non-revised index of Consumer Prices for All Urban Consumers (CPI-U)” as defined in the 2008 ISDA Inflation Derivatives Definitions.
The following Gibson Dunn attorneys assisted in preparing this update: Jeffrey Steiner, Adam Lapidus, Marc Aaron Takagaki, Hayden McGovern, Karin Thrasher, and Alice Wang*.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these developments. Please contact the Gibson Dunn lawyer with whom you usually work, any member of the firm’s Derivatives practice group, or the following practice leaders and authors:
Jeffrey L. Steiner, Washington, D.C. (202.887.3632, jsteiner@gibsondunn.com)
Michael D. Bopp, Washington, D.C. (202.955.8256, mbopp@gibsondunn.com)
Michelle M. Kirschner, London (+44 (0)20 7071.4212, mkirschner@gibsondunn.com)
Darius Mehraban, New York (212.351.2428, dmehraban@gibsondunn.com)
Jason J. Cabral, New York (212.351.6267, jcabral@gibsondunn.com)
Adam Lapidus, New York (212.351.3869, alapidus@gibsondunn.com )
Stephanie L. Brooker, Washington, D.C. (202.887.3502, sbrooker@gibsondunn.com)
William R. Hallatt, Hong Kong (+852 2214 3836, whallatt@gibsondunn.com )
David P. Burns, Washington, D.C. (202.887.3786, dburns@gibsondunn.com)
Marc Aaron Takagaki, New York (212.351.4028, mtakagaki@gibsondunn.com )
Hayden K. McGovern, Dallas (214.698.3142, hmcgovern@gibsondunn.com)
Karin Thrasher, Washington, D.C. (202.887.3712, kthrasher@gibsondunn.com)
Alice Yiqian Wang, Washington, D.C. (202.777.9587, awang@gibsondunn.com)
*Alice Wang, a law clerk in the firm’s Washington, D.C. office, is not admitted to practice law.
© 2025 Gibson, Dunn & Crutcher LLP. All rights reserved. For contact and other information, please visit us at www.gibsondunn.com.
Attorney Advertising: These materials were prepared for general informational purposes only based on information available at the time of publication and are not intended as, do not constitute, and should not be relied upon as, legal advice or a legal opinion on any specific facts or circumstances. Gibson Dunn (and its affiliates, attorneys, and employees) shall not have any liability in connection with any use of these materials. The sharing of these materials does not establish an attorney-client relationship with the recipient and should not be relied upon as an alternative for advice from qualified counsel. Please note that facts and circumstances may vary, and prior results do not guarantee a similar outcome.
Continue Reading
-

Matsuzawa Named To Walter Camp All-America Team
University of Hawai’i placekicker Kansei Matsuzawa was named to the Walter Camp All-America first team,…
Continue Reading
-

How the soundtrack was made
Mark SavageMusic correspondent
 20th Century Studios
20th Century StudiosAvatar: Fire and Ash is predicted to be one of the year’s highest-grossing movies It’s no secret the Avatar films are a gigantic technical feat – pushing the boundaries of cinematography, animation…
Continue Reading
-
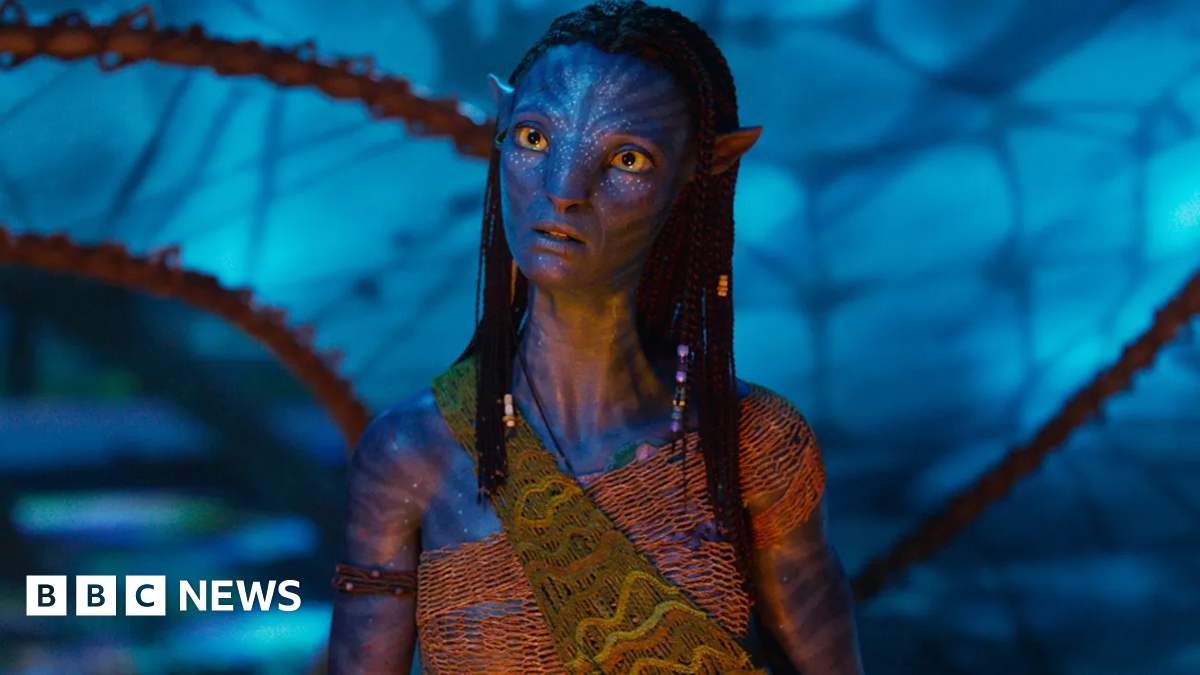
Avatar: Fire and Ash: How the soundtrack was made
The opposite is true on Avatar.
“Jim [Cameron] still believes that the good things take time. And as a composer, having that ability to refine and to make something special is something that is rare these days.”
The director also went to great…
Continue Reading
