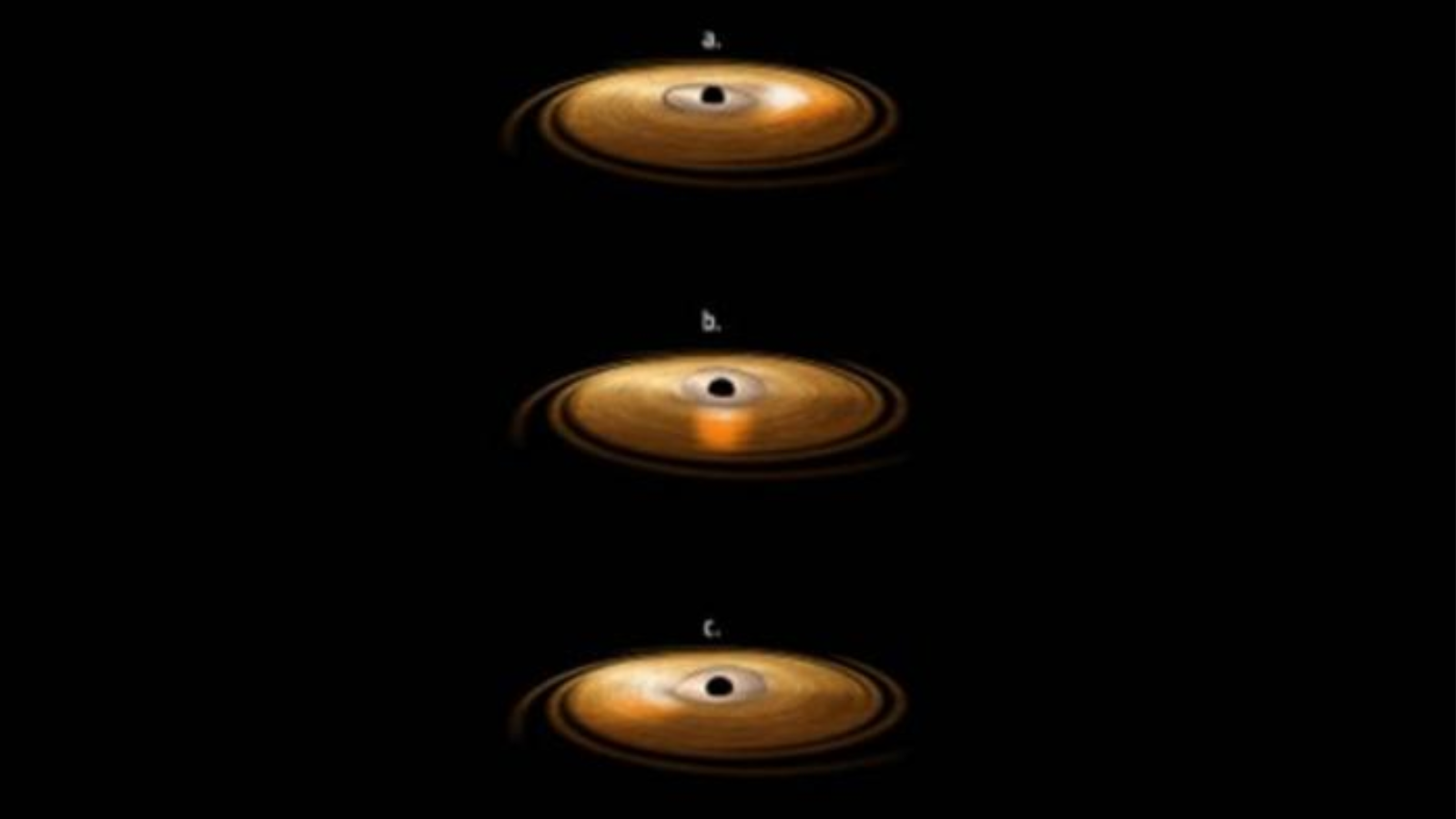
Astronomers have observed a star wobbling in its orbit around a ravenous supermassive black hole that is ripping it apart and feasting on its stellar material. The observation is evidence of a rare and elusive phenomenon called the…
Author: admin
-
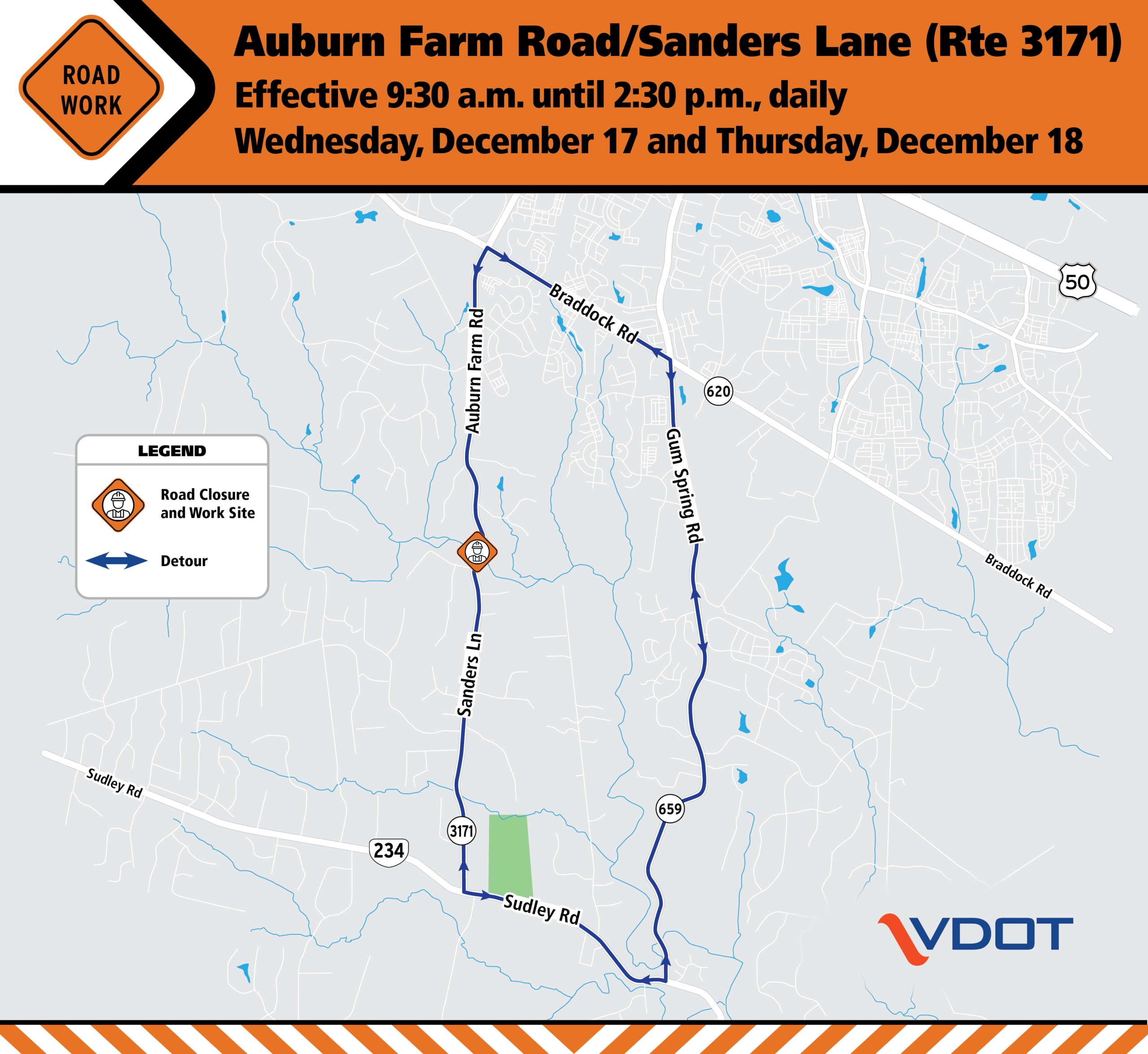
Northern Virginia District | Auburn Farm Road/Sanders Lane daytime closures at Loudoun/Prince William County line Dec. 17 & 18
Northern Virginia District | Auburn Farm Road/Sanders Lane daytime closures at Loudoun/Prince William County line Dec. 17 & 18 | Virginia Department of Transportation
Detour in place during Bull Run bridge guardrail installation
Last updated: December 12, 2025
Continue Reading
-

Japan, U.S. defense ministers share serious concern about China’s actions
Japanese Defense Minister Shinjiro Koizumi and his U.S counterpart, Pete Hegseth, held telephone talks on “increasingly severe security situation in the Indo-Pacific region, including the radar incident,”…
Continue Reading
-

How Smart Cruise Ship Elevators Prevent Crowds & Save Time
One of the first things Sailors notice on Virgin Voyages — often without realizing they’re noticing it — is how gracefully the ship moves them. You drift from cabin to brunch, from yoga to The Dock, from sun deck to Scarlet Night, and somehow… it always feels smooth, intuitive, and unhurried.
That’s no accident. It’s design.
And one of the biggest unsung heroes of that seamless experience? The elevators.
They don’t just go up and down — they help shape how Sailors travel through the ship in a way that keeps everything feeling beautifully calm.
Let’s take a ride inside the thinking behind it all.
The elevators don’t react — they flow with the ship
In many buildings, elevators simply respond. On Virgin Voyages, they feel like part of the ship’s rhythm.
Our elevator systems anticipate movement patterns — mornings drifting upward toward breakfast, afternoons settling around the pool decks, evenings expanding outward to restaurants and venues. While they aren’t “predictive AI,” they are programmed to handle shifting needs throughout a voyage, adapting efficiently to real Sailor flow.
The result? Your ride feels smooth, quick, and surprisingly effortless.
✨ Design Callout:
Great design doesn’t call attention to itself. It simply works — and makes the whole experience feel better.Intentional elevator placement helps the whole ship breathe
Virgin Voyages’ ships are designed around flow — the natural rhythm of how Sailors move through spaces. That’s why you’ll find elevator banks intentionally placed in the forward, midship, and aft sections of the ship.
This creates:
- multiple access points
- natural distribution of movement
- intuitive navigation
- shorter walks to vertical transportation
It’s not something most Sailors consciously notice — but they feel the ease. You don’t search for elevators. You just find them exactly where you need them.
See how our ships are designed.
Elevators respond to the voyage’s natural rhythm
Every voyage has its own daily choreography — and the elevators adjust accordingly.
While the system doesn’t read minds or use futuristic predictive tech, it does manage movement efficiently by responding dynamically to higher-demand times and deck-to-deck flow. This subtle adaptability helps keep movement around the ship feeling relaxed rather than rushed.
🌬️ Callout:
When everything feels calm, it’s because the design is doing its job — not because the ship is empty.Even the elevator ambiance supports the onboard mood
Virgin Voyages is intentional about ambiance — lighting, materials, sound — and the elevators are no exception.
Inside each cab, you’ll find:
- soft, warm lighting in the morning + more electric lighting toward night
- smooth ride feel
- elegant finishes that echo the ship’s design language
Together, they create the relevant vibe instead of the usual “transportation mode” feeling of city elevators.
💡 Virgin Tip:
Look at the way the elevator lighting reflects on the doors as they open — it’s a surprisingly soothing moment of design poetry.Forward, midship & aft elevators = effortless navigation
Because the ship has three elevator banks, Sailors naturally spread out — which keeps each ride comfortable and minimizes wait time without needing to over-engineer the system.
Choosing the right bank becomes a kind of secret Sailor superpower:
- Forward: spa, lounges, The Red Room, entertainment
- Midship: central access, pools, dining, gym
- Aft: The Dock, The Galley, wake views, outdoor lounging
Knowing this makes your movement through the ship feel even easier.
🧭 Sailor Hack:
Heading to Scarlet Night or dinner? Elevator mirrors have the best lighting for quick outfit checks — better than your cabin.The beauty of it all? You hardly notice it
The true sign of thoughtful design is when a complex system feels simple. Virgin Voyages elevators don’t ask for attention — they quietly support the entire onboard journey. They help keep the ship feeling spacious, relaxed, intuitive, and delightfully easy to explore. You move without stress. You arrive without effort. You float — through the ship, just as you float on the water.
Ready to experience a ship that moves with you?
Explore Virgin Voyages sailings.
Continue Reading
-
Tabernacle Choir and Orchestra’s 2025 Christmas concert – Church News
With both festive and worshipful music and a story about a unique Christmas Eve in space, The Tabernacle Choir and Orchestra at Temple Square’s 2025 Christmas concert celebrated the season of Jesus Christ’s birth and the invitation to seek…
Continue Reading
-

Which Christmas movies have been most widely seen — and loved
Whether they’re decades-old classics or recent releases, dozens of Christmas movies are widely enjoyed by Americans, new YouGov polling finds. The vast majority (79%) of Americans say they love or like holiday or Christmas movies, and most (64%)…
Continue Reading
-

Prostate Cancer Screening: Tests & Diagnosis from Fred Hutch
Good prostate cancer screening tests have led to early diagnosis in about 80% of men with the disease. According to the American Cancer Society, all of these men survive at least five years. Screening alone is credited for one-third of the…
Continue Reading
-

Iowa State Wraps 2025 With ISU Holiday Invite
AMES, Iowa – Two Cyclones put up top 10 program performances as Iowa State ended 2025 with the ISU Holiday Invite.
In the 60m hurdles final, freshman Emma Havghurst got off to a fast start tying the 10th-fastest ISU performance in her first…
Continue Reading
-

Marie Antoinette Style and the Jewels That Define It
< Historic Diamonds / Royal Stories
Only months remain at the V&A exhibition, Marie Antoinette Style, to experience the opulent legacy of history’s most stylish queen.
Published: December 12,…
Continue Reading
-

Grassroots First Nations organisation flags concern for at-risk youth under social media ban
Prime Minister Anthony Albanese says Australia’s landmark social media ban is about protecting the wellbeing of children.
But when ABC News attended a drop-in youth centre working with some of the country’s most vulnerable children and teenagers,…
Continue Reading