When an email about natural disasters and wildfire risk mentions the name of a local suburb, people pay more attention.
This local wording makes people about twice as likely to open an email link to learn more, according to a new study.

When an email about natural disasters and wildfire risk mentions the name of a local suburb, people pay more attention.
This local wording makes people about twice as likely to open an email link to learn more, according to a new study.
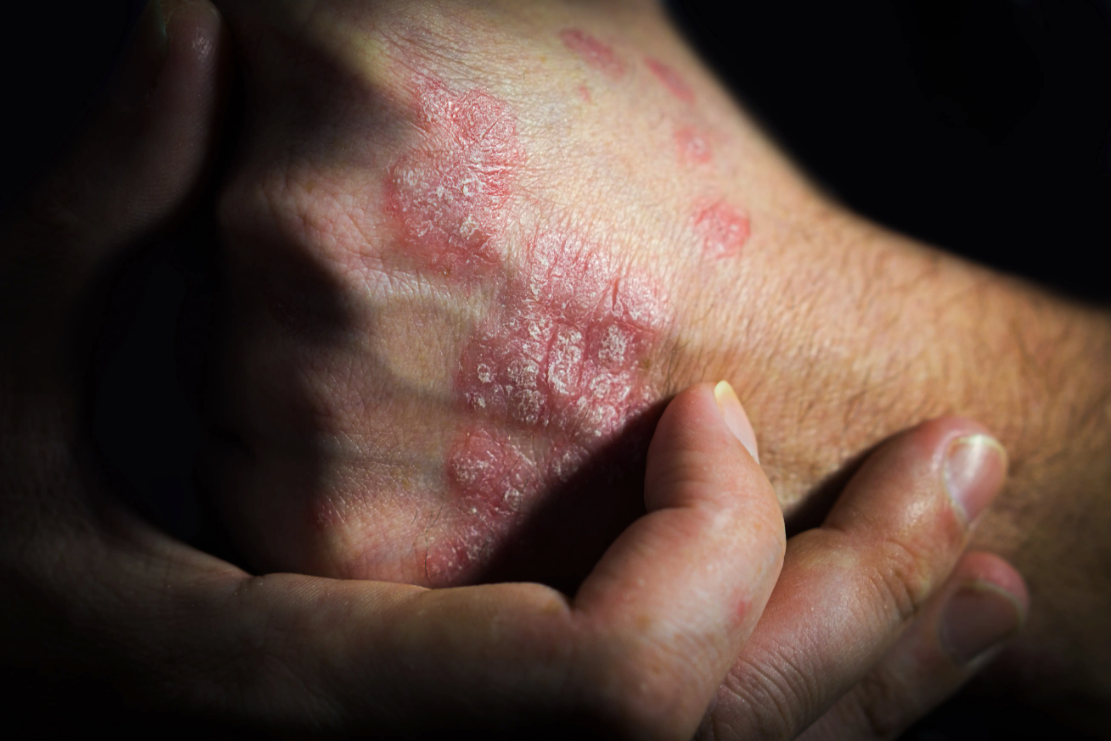
Bimekizumab, a dual IL-17A/IL-17F inhibitor used for
This retrospective analysis is published in
“Given the limited time since its market approval, there is a scarcity of long-term safety data based on real-world studies,” wrote the researchers of the study. “To address this gap, we utilized the FAERS database to identify and analyze bimekizumab-related AEs, thereby enhancing our understanding of its safety profile in routine clinical practice.”
In the US, bimekizumab was first authorized in October 2023 for adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, based on phase 3 trial data demonstrating high levels of skin clearance.2 Since then, US approvals have expanded to include active psoriatic arthritis, active nonradiographic axial spondyloarthritis with objective signs of inflammation, and active ankylosing spondylitis.3 These indications position bimekizumab as one of the first dual IL-17A/IL-17F inhibitors available for a range of immune-mediated inflammatory disorders.
This study used a retrospective pharmacovigilance design to evaluate AEs associated with bimekizumab using data from the FAERS.1 All reports submitted between Q3 2021 and Q4 2024 were extracted and screened for cases listing bimekizumab as the primary suspect drug. Analyses were conducted to identify statistically significant safety signals across system organ classes, using established metrics to compare the frequency of reported events with those of the full FAERS database. Time-to-onset analyses were also performed to characterize when adverse events occurred following drug initiation.
Among the 6,037,398 AE reports submitted to FAERS during the study period, 2780 were associated with bimekizumab. The researchers identified 70 significant adverse event signals across 11 System Organ Classes. Many aligned with the drug’s known safety profile, including injection site pain, oral candidiasis, and esophageal candidiasis. However, 29 previously unreported signals also emerged, such as cellulitis, lower respiratory tract infections, Staphylococcus infections, immunodeficiency, and Mycoplasma pneumonia.
Time-to-onset analysis showed a median (IQR) onset of 29 (0, 84.75) days, indicating that many events occurred within the first several weeks of therapy.
However, the researchers acknowledged several limitations. First, FAERS reports are often incomplete, leading to missing data that may bias both AE patterns and time-to-onset findings. Because information on patients taking bimekizumab without AEs was unavailable, true incidence rates could not be calculated. Additionally, the presence of confounders, such as concomitant drugs or underlying conditions, further limited interpretability of this retrospective study, so controlled clinical studies are needed to confirm these safety signals.
Despite these findings, the researchers believe these findings confirm established risks while revealing additional safety signals that warrant further clinical attention in bimekizumab.
References
1. Lin X, Guo L, Lin T, et al. Disproportionality analysis of adverse events associated with bimekizumab: a real-world study based on FDA Adverse Event Reporting System (FAERS) Database. Naunyn Schmiedebergs Arch Pharmacol. doi:10.1007/s00210-025-04894-2
2. Myshko D. Bimekizumab-bkzx, the newest psoriasis treatment, is now available. AJMC®. November 30, 2023. Accessed December 11, 2025.
3. McNulty R. FDA approves bimekizumab for psoriatic arthritis, nonradiographic axSpA, ankylosing spondylitis. AJMC. September 23, 2024. Accessed December 11, 2025.
For generations, school posters have sorted the Solar System into tidy…
Press release as PDF
After a review process led by the IBSF AIN Task Force and with the support of Sportradar, the IBSF published on 12 December the list of individual neutral athletes (AIN) who may participate in IBSF events with immediate…

Former champion Rob Cross is safely through to the second round of the PDC World Championship after a comfortable 3-0 win over Norway’s Cor Dekker.
The Englishman, who triumphed on his debut at Alexander Palace in 2018, has endured a difficult…


Acute myocardial infarction (AMI), also known as myocardial infarction, remains one of the leading causes of mortality and disability worldwide, posing a severe threat to human health.1 AMI is characterized by acute occlusion of the…
“The fact that patients with inflammatory bowel disease (IBD) often experience symptoms like abdominal pain even during phases of disease remission suggests that mechanisms other than acute inflammatory processes contribute to the…

Behind the polished exterior of many modern buildings sit outdated systems with vulnerabilities waiting to be found
12 Dec 2025
•
,
3 min. read

“A…