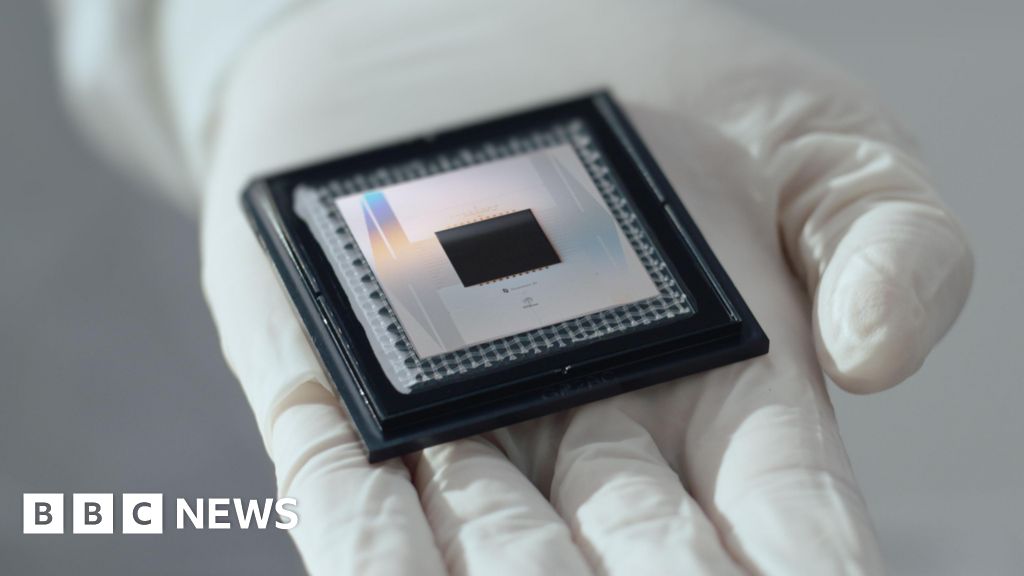
Google has announced plans to team up with the UK to invite researchers to come up with uses for the tech giant’s state-of-the-art quantum chip Willow.
It is one of several firms competing to develop a powerful quantum computer – which is seen as an exciting new frontier in the future of computing.
Researchers hope they will be able to crack problems in fields such as chemistry and medicine which are impossible for current computers to solve.
Professor Paul Stevenson of the University of Surrey – who had no involvement with the agreement – told the BBC it was “great news for UK researchers”.
The collaboration between Google and the UK’s national lab for quantum computing means more researchers will get access to the technology.
“The new ability to access Google’s Willow processor, through open competition, puts UK researchers in an enviable position,” said Prof Stevenson.
“It is good news for Google, too, who will benefit from the skills of UK academics.”
Quantum devices work in a fundamentally different way to the computers powering our smartphones and laptops, solving problems using technologies based on the science of particle physics
But the full potential of the technology has yet to be realised and the machines that currently exist have few practical applications and most are experimental.
It is hoped giving UK researchers access to Willow would help “uncover new real world applications”.
Scientists will be able to submit proposals describing how they intend to use the chip, and they will work with experts from Google and the UK quantum lab to design and conduct experiments.
When it was unveiled in 2024, Google’s Willow chip was seen as a significant step forward in the field.
Rival firms including Amazon and IBM are also developing their own tech.
The UK has a significant quantum industry. Quantinuum, which has headquarters in Cambridge and Colorado, US, reached a $10bn (£7.45bn) valuation in September.
Announcements of new developments from firms throughout 2025 have led some experts to believe powerful machines capable of having real-world impact will be developed within a decade.
Dr Michael Cuthbert, Director at the National Quantum Computing Centre (NQCC) said the partnership would “accelerate discovery”.
He said the cutting edge science it would support could ultimately lead to quantum computing being used in areas such as “life science, materials, chemistry, and fundamental physics”.
The NQCC already hosts seven quantum computers from British-based firms such as Quantum Motion, ORCA and Oxford Ionics.
The government says it is committing £670m to support the tech, which is a priority area in the UK’s Industrial Strategy.
Officials believe quantum could contribute £11 billion to the UK economy by 2045.