- Exclusive: U.S. Marines fired on protesters storming consulate in Karachi, officials say Reuters
- Security Alert: U.S. Mission to Pakistan (March 2, 2026) U.S. Embassy & Consulates in Pakistan (.gov)
- US Marines fired on protesters storming…
Author: admin
-
Exclusive: U.S. Marines fired on protesters storming consulate in Karachi, officials say – Reuters
-
Huawei Launched Next-Generation Voice Virtual Agents for Its Artificial Intelligence Contact Center, Defining a New Paradigm for Semantic Dialogue
[Barcelona, Spain, March 2, 2026] During MWC Barcelona 2026, Huawei launched next-generation voice virtual agents for its Artificial Intelligence Contact Center (AICC) with hyper-human voice interaction capabilities. It aims to provide more efficient and intelligent customer service experiences for industries including carriers, finance, government, and transportation etc., improving the self-service resolution rate by 20%.
With the rapid evolution of large language models, enterprises have higher requirements for optimizing customer experience, improving operational efficiency, and achieving intelligent upgrades. Huawei AICC continuously innovates, building a next-generation of voice virtual agent solution centered on
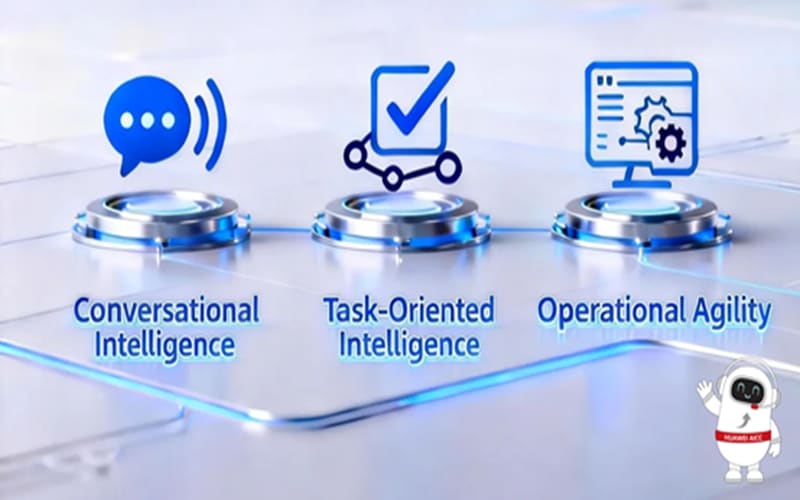
“domain-specific models + intelligent agents” to enable virtual agents with “Conversational Intelligence, Task-Oriented Intelligence, Operational Agility”. This solution features three key capabilities:
Voice Virtual Agents three key capabilities
- Conversational Intelligence: Powered by large language models fine-tuned for the customer service scenarios, these virtual agents learn from the best practices of top-performing customer service representatives. They generate hyper-human responses in real-time using TTS (Text-to-Speech), with a voice quality that closely mimics human intonation and tone. The immersive intelligent interruption and intelligent response capabilities create a smoother human-machine interaction, with a user experience MOS (Mean Opinion Score) exceeding 4.5, reaching an excellent level.
- Task-Oriented Intelligence: Based on domain-specific large models and Huawei’s self-developed CAE (Conversational Agent Engine), these virtual agents provide high business process compliance, precise intent recognition, tool invocation, and secure, controllable multi-turn dialogue capabilities for complex tasks. This enables the virtual agents to move beyond simple “chatting” and achieve true end-to-end “closed-loop” problem resolution for users.
- Operational Agility: Through visualized and no-code SOP(Standard Operating Procedure) orchestration, these virtual agents support low-barrier business operations. AI-assisted SOP process mining and continuous accumulation facilitate the rapid rollout of new scenarios and efficient operations and maintenance (with a TTM of less than two weeks). The performance of business processes can be monitored, and online application optimization can be done with a “what you see is what you get” approach.
The launch of this next-generation of voice virtual agents marks a significant upgrade in contact centers, transitioning from a single semantic interaction to an end-to-end closed-loop. By deeply integrating domain-specific large models with agent technology, this innovation achieves a human-like leap in interaction experience. Additionally, the agile SOP operational system effectively breaks down the barriers between technology and business. This is not only a quantitative improvement in customer service efficiency but also a qualitative transformation toward “delivering definitive value” as enterprises advance their digital transformation. Moving forward, Huawei will continue collaborating with global customers and partners to expedite the seamless integration of AI technologies into industry scenarios. This will empower contact centers to enhance customer experiences, boost operational efficiency, and drive revenue growth.
MWC Barcelona 2026 will be held from March 2 to March 5 in Barcelona, Spain. During the event, Huawei will showcase its latest products and solutions at stand 1H50 in Fira Gran Via Hall 1.
The era of agentic networks is now approaching fast, and the commercial adoption of 5G-A at scale is gaining speed. Huawei is actively working with carriers and partners around the world to unleash the full potential of 5G-A and pave the way for the evolution to 6G. We are also creating AI-Centric Network solutions to enable intelligent services, networks, and network elements (NEs), speeding up the large-scale deployment of level-4 autonomous networks (AN L4), and using AI to upgrade our core business. Together with other industry players, we will create leading value-driven networks and AI computing backbones for a fully intelligent future.
For more information, please visit: https://carrier.huawei.com/en/minisite/events/mwc2026/
Continue Reading
-

Bruce Campbell, ‘Evil Dead’ actor, reveals cancer diagnosis
Bruce Campbell attends the Hysteria! press room during New York Comic Con 2024 at The Jacob K. Javits Convention Center on October 17, 2024 in New York City. (Credit: Eugene Gologursky/Getty Images for ReedPop)
Actor Bruce Campbell…
Continue Reading
-

TTAP delegation visits Iranian embassy – Newspaper
ISLAMABAD: A delegation of the opposition alliance, Tehreek Tahafuz Ayeen-i-Pakistan (TTAP), on Monday visited the Iranian embassy to offer condolence and express solidarity with the neighbouring country, which is under attacks by US and…
Continue Reading
-

Cybercrime investigation unit approved for Punjab – Newspaper
LAHORE: Chief Minister Maryam Nawaz on Monday approved the establishment of the province’s first cybercrime investigation unit and made e-tagging mandatory for all vehicles, including motorcycles.
Presiding over a special meeting held to…
Continue Reading
-

Rival lawyer groups clash over LHCBA poll results in Lahore – Pakistan
LAHORE: The members of the two rival lawyer groups – one led by Hamid Khan and the other by Ahsan Bhoon — came face to face during a meeting convened on Monday to resolve the dispute over the Lahore High Court Bar Association (LHCBA) election…
Continue Reading
-

PTI moves IHC for shifting Imran to hospital – Newspaper
ISLAMABAD: The Islamabad High Court (IHC) was moved on Monday with a plea seeking the immediate shifting of incarcerated PTI founder Imran Khan to Shifa International Hospital for specialised eye treatment, alleging that jail authorities have…
Continue Reading
-
Threat from Iran conflict spreads across Middle East in 72 hours – The Washington Post
- Threat from Iran conflict spreads across Middle East in 72 hours The Washington Post
- US-Israel attacks on Iran: Death toll and injuries live tracker Al Jazeera
- ‘They hit so hard the house was shaking’: Iranians describe impact of US-Israel…
Continue Reading
-
State Department Urges Americans to Leave Middle East Now – Bloomberg
- State Department Urges Americans to Leave Middle East Now Bloomberg
- US urges citizens to immediately leave over a dozen Middle East countries Al Jazeera
- Iran-US war live: US embassy alarms sounded as Israel strikes Tehran and Beirut The…
Continue Reading
-

Melania Trump urges protecting children’s education at UN after Iran school strike | US news
Melania Trump became the first spouse of a sitting world leader to preside over the UN security council on Monday, calling on member states to protect children’s access to education days after Iranian state media reported that an airstrike
Continue Reading