From 10 December, the experience of the Games is ever closer to the public and spectators. Tickets MilanoCortina2026 is now available, the only official app to access all the competition venues and iconic locations that will host the Olympic and…
Author: admin
-

Strict security in place around Adiala Jail ahead of PTI founder’s meeting with lawyers – Pakistan
Authorities in Rawalpindi shut down shops, markets and petrol pumps around Adiala Jail on Tuesday as part of security measures ahead of PTI founder Imran Khan’s meeting with lawyers.
Educational institutions in the vicinity have also been…
Continue Reading
-
TY1: new experimental drug restores tissue after heart attack
Scientists have developed an experimental RNA-based drug, TY1, that repairs DNA, reduces scar tissue and could lead to new treatments for heart attacks and autoimmune diseases.

Cedars-Sinai researchers have created an…
Continue Reading
-
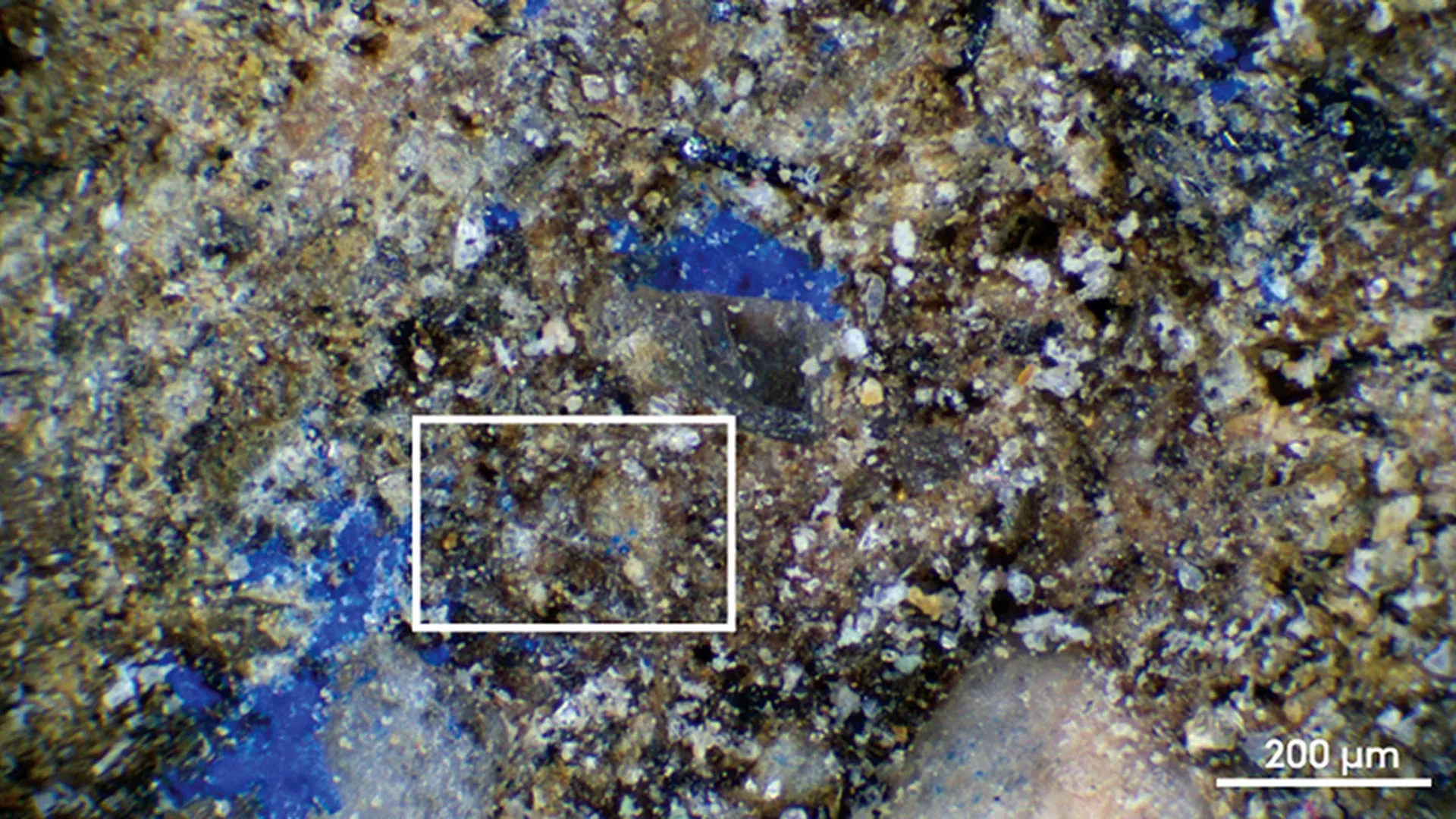
Stunning blue pigment on a 13,000-year-old artifact surprises scientists
At the Final Paleolithic site of Mühlheim-Dietesheim in Germany, researchers from Aarhus University identified faint blue traces on a stone artifact that dates to roughly 13,000 years ago. After applying a variety of advanced scientific…
Continue Reading
-

Ben & Jerry’s brand could be destroyed, says co-founder
Ben & Jerry’s will be destroyed as a brand if it remains with parent company Magnum, the company’s co-founder Ben Cohen has told the BBC.
His remarks are the latest in a long-running spat between the ice cream brand and its parent company over its ability to express its social activism and the continued independence of its board.
It comes on the day that the Magnum Ice Cream Company (TMICC) started trading on the European stock market – spinning off from owner Unilever.
A spokesperson for Magnum said the firm wanted to build and strengthen Ben & Jerry’s “powerful, non-partisan values-based position in the world”.
Ben & Jerry’s was sold to Unilever in 2000 in a deal which allowed it to retain an independent board and the right to make decisions about its social mission.
Since the sale there have been deepening clashes between the Vermont-based brand and Unilever, with this conflict now inherited by Magnum.
In 2021, Ben & Jerry’s refused to sell its products in areas occupied by Israel, resulting in its Israeli operation being sold by Unilever to a local licensee, and in October, Ben Cohen said it was prevented from launching an ice cream which expressed “solidarity with Palestine”.
Last month, ahead of its spin off from Unilever, Magnum said the chair of Ben & Jerry’s board Anuradha Mittal, who has held the position since 2018, “no longer meets the criteria to serve” – saying this was the result of an internal audit.
A spokesperson for Magnum said it had found “a series of material deficiencies in financial controls, governance and other compliance policies, including conflicts of interest”.
“So far, the trustees have not fully addressed the deficiencies identified,” they said.
In a statement to Reuters, Ms Mittal said: “The so-called audit of the foundation was a manufactured inquiry – engineered to attempt to discredit me.
“It is important to understand that this is not simply an attack on me as chair. It is Unilever’s attempt to undermine the authority of the Board itself.”
The BBC has contacted Ben & Jerry’s to request this statement.
Mr Cohen said Magnum “has no standing to determine who the chair of the independent board should be”.
“Therefore, by trying to [change the chair of the board], I would say that Magnum is not fit to own Ben & Jerry’s,” he added.
Mr Cohen called for either the business to be “owned by a group of investors that support the brand and want to encourage the values” or for Magnum to make a “180 degree turn around and say they support the chairman of the independent board”.
Ahead of the spin off on Monday, news agency Reuters reported that Ms Mittal said she had no plans to step down from the board.
Ben Cohen remains an employee of Ben & Jerry’s and the brand’s most high-profile spokesperson.
He told the BBC he feared under the current ownership the ice cream maker’s “loyal” followers would be lost for good.
“If the company continues to be owned by Magnum, not only will the values be lost, but the essence of the brand will be lost,” he said.
On Sunday, Magnum’s chief executive Peter ter Kulve told the Financial Times the Ben & Jerry’s founders were in their seventies and “at a certain moment they need to hand over to a new generation”.
Jerry Greenfield, Mr Cohen’s co-founder, left the ice cream maker in September after almost half a century at the firm – citing concerns about the stifling of its social mission.
“It’s absurd,” said Mr Cohen.
“This is about values and abiding by a legally binding agreement.”
Mr Cohen added investors in Magnum were being asked to pay a premium for the Ben & Jerry’s brand “because it has such a loyal following”.
“As they destroy Ben and Jerry’s values, they will destroy that following and they will destroy that brand,” he said.
“It’ll become just another piece of frozen mush that just going to lose a lot of market share.”
A spokesperson for Magnum said Ben & Jerry’s was “not for sale” and it had “always respected” the brand’s commitment to continue its “social mission”.
The demerger of Unilever’s ice cream business saw primary shares in Magnum open at €12.20 (£10.66) – down on the expected €12.80 (£11.18) reference price set by the EuroNext exchange in Amsterdam. But it bounced back up by 1.3% at close of trading.
The spin off means Magnum is now the world’s biggest standalone ice cream business.
Continue Reading
-

Kate Winslet Directorial Debut ‘Goodbye June’ Interview
EXCLUSIVE: Kate Winslet has made it. Her directorial debut feature, the heart-warming, and heart-breaking, Goodbye June, which the Oscar-, Emmy- and BAFTA Award-winning star shot from a screenplay by her son, Joe Anders, is to be released in…
Continue Reading
-

Are pumpkin seeds really safe? Ayurveda expert warns too many may cause indigestion, weight gain, and low blood pressure
Pumpkin seeds have quietly earned a spot on the list of popular ‘superfoods’, thanks to their rich dose of magnesium, zinc, antioxidants, and healthy fats. Many people sprinkle them on salads, blend them into smoothies, or eat them as a…
Continue Reading
-

Global Fashion Agenda Grows Efforts to Combat Textile Waste through New Initiative in Türkiye
Today, Global Fashion Agenda (GFA) announced the launch of the Circular Fashion Partnership: Türkiye, a new initiative that aims to support the development of a circular textile system in the country by capturing and recycling post-industrial textile waste. Announced during Sustainability Talks Istanbul, the partnership is led by GFA in collaboration with national lead Rematters, supported by implementation partners Reverse Resources, Closed Loop Fashion, and Circle Economy Foundation and funded by H&M Foundation.
Set to commence in early 2026, the Circular Fashion Partnership: Türkiye aims to establish textile waste management systems within factories, enhance traceability through digital tools, and connect manufacturers with recyclers to ensure higher-value recovery of post-industrial textile waste. The programme will also provide supplier support on compliance with evolving policy frameworks and foster national collaboration to drive systemic change. GFA is now calling on brands producing in Türkiye to participate in the programme.
As one of the world’s leading apparel manufacturing hubs, Türkiye is uniquely positioned to scale textile-to-textile recycling due to its vertically integrated industry, proximity to the EU, and increasing regulatory pressure to reduce waste and emissions. The Circular Fashion Partnership: Türkiye will build on these strengths by developing scalable models for improved waste segregation, fibre-to-fibre recycling, and domestic recovery routes that reduce dependency on virgin materials and landfill.
The programme is part of the Global Circular Fashion Forum (GCFF), a wider initiative led by Global Fashion Agenda to advance post-industrial textile recycling through local partnerships in manufacturing regions. Building on successful implementation in Bangladesh, Cambodia, and Indonesia, the Circular Fashion Partnership: Türkiye becomes the fourth national programme to deploy this model — which has already digitally traced over 21,000 tonnes of textile waste and connected more than 100 factories and 20 global brands to recycling partners across its programmes. The locally owned and led partnership in Türkiye will be customised to the regional context, while drawing on best practices from other countries. Throughout 2026, the Circular Fashion Partnership: Türkiye will engage stakeholders across the value chain via targeted activities including on-site waste management assessments, training and capacity building through a Train-the-Trainer model, recycling pitch sessions and matchmaking events, as well as roundtables and policy dialogues with key national actors. In doing so, the partnership aims to support Türkiye in futureproofing its textile ecosystem, unlock economic value from waste, and contribute to a just, circular transition in one of the industry’s most influential sourcing regions.
Continue Reading
-

Treasury yields little changed as markets await jobs report
U.S. Treasury yields were little changed as investors anticipate jobs and employment data coming out later in the day.
The 10-year Treasury yield was relatively flat at 4.178%. The 30-year Treasury yield slid by less than one basis point to 4.81%, as did the 2-year Treasury yield to 4.781%.
One basis point is equivalent to 0.01%, and yields and prices share an inverse relationship.
The Job Openings and Labor Turnover Summary (JOLTS) Job Openings report for October is slated to come in at 7.15 million, according to LSEG estimates.
Market participants are expecting that the Federal Reserve will lower its benchmark interest rate at its final meeting of the year.
“We believe a December rate cut will work to support equity markets and credit quality,” Eastspring Investments wrote in a note.
If Fed Chair Jerome Powell suggests that he views the Fed is now in a good enough place to skip the next few meetings to assess the economy, this would likely reinforce the current stability of the U.S. dollar and keep Treasury yields in their recent range, the economists added.
“In contrast, a more dovish message – keeping the prospect of a January rate cut alive – would likely weaken the USD and lead to a bearish steepening of the US Treasury curve,” the analysts noted.
Continue Reading
-

Whoops! A Galaxy Z TriFold prototype shows four rear cameras
Samsung’s first tri-folding smartphone, the Galaxy Z TriFold, was recently announced. It’ll be available later this week in South Korea and sometime early next year in the USA. This phone-tablet hybrid has three…
Continue Reading
