- Meta pledge to use less personal data for ads gets EU nod, avoids daily fines Reuters
- Facebook and Instagram will let European users see fewer personal ads The Verge
- Meta must remove dark patterns to comply with DMA requirements, consumer group says MLex
- EU Acknowledges Meta’s Undertaking To Offer EU Users Ad Choice On Facebook, Instagram Nasdaq
- Meta proposal for less data sharing is approved by European Commission The Record from Recorded Future News
Author: admin
-
Meta pledge to use less personal data for ads gets EU nod, avoids daily fines – Reuters
-

Nanotyrannus Represents Distinct Species of Tyrannosaurid Dinosaur, New Research Confirms
Nanotyrannus lancensis — long thought by many to be a teenage Tyrannosaurus rex — was in fact a fully mature, distinct species of smaller tyrannosaurid, according to a team of U.S. paleontologists who analyzed the ceratobranchial (hyoid…
Continue Reading
-
Streetlights, manhole covers: Each UC to get Rs0.1m per month – Business Recorder
- Streetlights, manhole covers: Each UC to get Rs0.1m per month Business Recorder
- People paying for municipal failure Dawn
- UCs to receive Rs100,000 monthly for manhole covers, streetlights The Express Tribune
- Karachi UCs to get Rs100,000 monthly…
Continue Reading
-
ADB warns of economic water scarcity in Pakistan – Business Recorder
- ADB warns of economic water scarcity in Pakistan Business Recorder
- Population growth puts strain on Pakistan’s water resources: ADB Dawn
- Infrastructure issues threaten Indus The Express Tribune
- Living with the Indus: Building the enabling…
Continue Reading
-
Israeli authorities raid UN Palestinian refugee agency's East Jerusalem compound – Reuters
- Israeli authorities raid UN Palestinian refugee agency’s East Jerusalem compound Reuters
- Israeli police raid UNRWA compound in East Jerusalem and replace UN flag with Israeli flag, head of the agency says CNN
- Netanyahu, Trump to discuss Gaza…
Continue Reading
-

More User-Engagement, More Complexity, More Options
The Peter B. Lewis building, designed by Frank Gehry,. The building is on the campus of Case Western Reserve University.
getty
Take a moment and read the obituaries that honored Frank Gehry’s life this past weekend. It is striking and informative…
Continue Reading
-

Implant Delivers Patterned Optogenetic Stimulation, Generates Artificial Perception in Mice
A wireless, ontogenetic device that sends information directly to the brain has been developed by a team at Northwestern University. The soft, flexible device sits under the scalp, on top of the skull, where it delivers precise…
Continue Reading
-

AI takes control in orbit, speeds ISS flying robot tasks by 60%
A toaster-sized robot just took a major step toward autonomous space navigation.
Stanford researchers have successfully demonstrated a machine-learning-based control system aboard the International Space Station (ISS) for the first…
Continue Reading
-
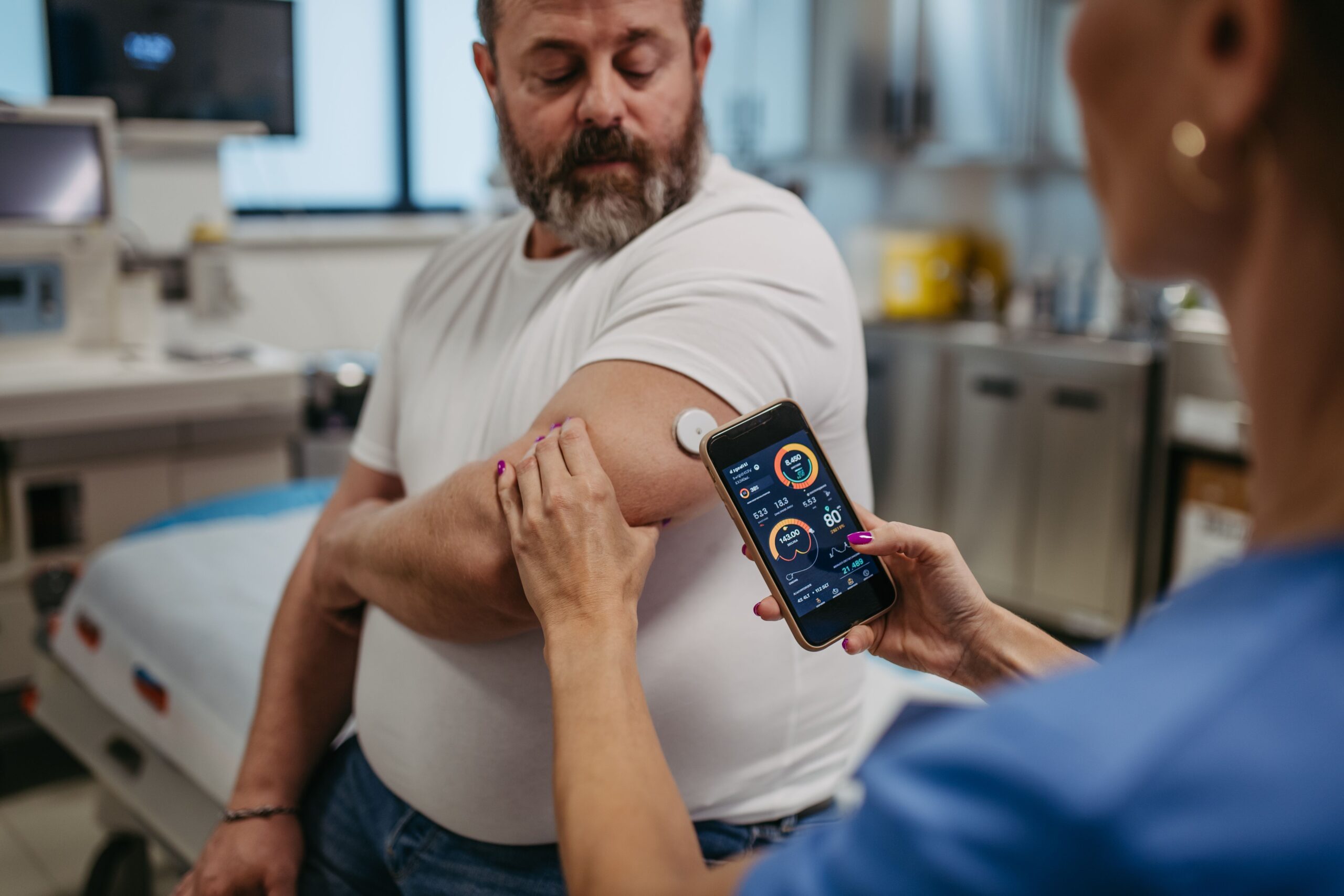
Pharmacist Strategies for Managing Comorbid Depression and Diabetes
Diabetes affects just under 12% of the population of the United States, and depression affects a little under 9%, but beneath these underwhelming statistics are some overwhelming correlations. Diagnosis with either of these conditions increases…
Continue Reading
-
Epidemiological Update: Pertussis (Whooping Cough) in the Americas Region – 8 December 2025 – PAHO/WHO
Organization (WHO) in 2024, representing a 5.8-fold increase compared with the number of cases reported in 2023 (n= 167,407 cases). The highest proportion of cases was recorded in the WHO Regions of the Western Pacific (n= 591,193 cases) and…
Continue Reading