British drama Adolescence has seen its lead stars all nominated for Golden Globe awards.
The drama, starring Stephen Graham, depicts the aftermath of the stabbing of a teenage girl – as a 13-year-old boy from her school is arrested…

British drama Adolescence has seen its lead stars all nominated for Golden Globe awards.
The drama, starring Stephen Graham, depicts the aftermath of the stabbing of a teenage girl – as a 13-year-old boy from her school is arrested…

Tuning the electronic properties of topological insulators presents a significant challenge for advanced materials science, but a new study demonstrates a method for precisely controlling these states. Matthew Rogers, Craig Knox, and Bryan…

ISLAMABAD (AP) — Pakistan’s newly appointed armed forces chief called on Afghanistan’s Taliban government on Monday to choose between maintaining ties with Islamabad or supporting the Pakistani…

…

A film full of craft, atmosphere, and character
Each time period has its own distinct character, enhanced by the choice of filming techniques and lenses. The film opens in black and white in 1870 and progresses through the warm Kodachrome tones of…

The ongoing pricing standoff, which began two months ago between BHP and China’s state-backed CMRG (China Minerals Resources Group), has added to uncertainty in the iron ore market. The standoff is part of China’s strategic push to exert greater influence over iron ore pricing and to increase the use of the yuan in contract settlements, reducing reliance on the US dollar.
CMRG was created by Beijing three years ago to shift leverage from major iron ore producers toward China, the world’s largest iron ore buyer.
Beijing has recently expanded its embargo on some BHP cargoes, ordering steel mills and traders to stop buying “jingbao fines”, a low-grade of iron ore that represents a small part of the miner’s exports to China. The ban follows an earlier halt on BHP’s “jimblebar fines”, a Pilbara iron ore grade and one of BHP’s most popular export types.
While the dispute is likely a negotiating tactic rather than a structural break, it heightens near-term volatility by disrupting trade flows and undermining confidence in China’s procurement approach. If unresolved, the impasse could drive a rerouting of some trade flows and force BHP to discount cargoes into alternative markets. For now, BHP has kept its full-year 2026 production guidance unchanged at 258-269 million tonnes.

Researchers have discovered how to inhibit the P2X4 receptor, a key protein linked to chronic pain, inflammation and certain cancers.

Scientists at the University of Bonn and University Hospital Bonn (UKB) have discovered a…
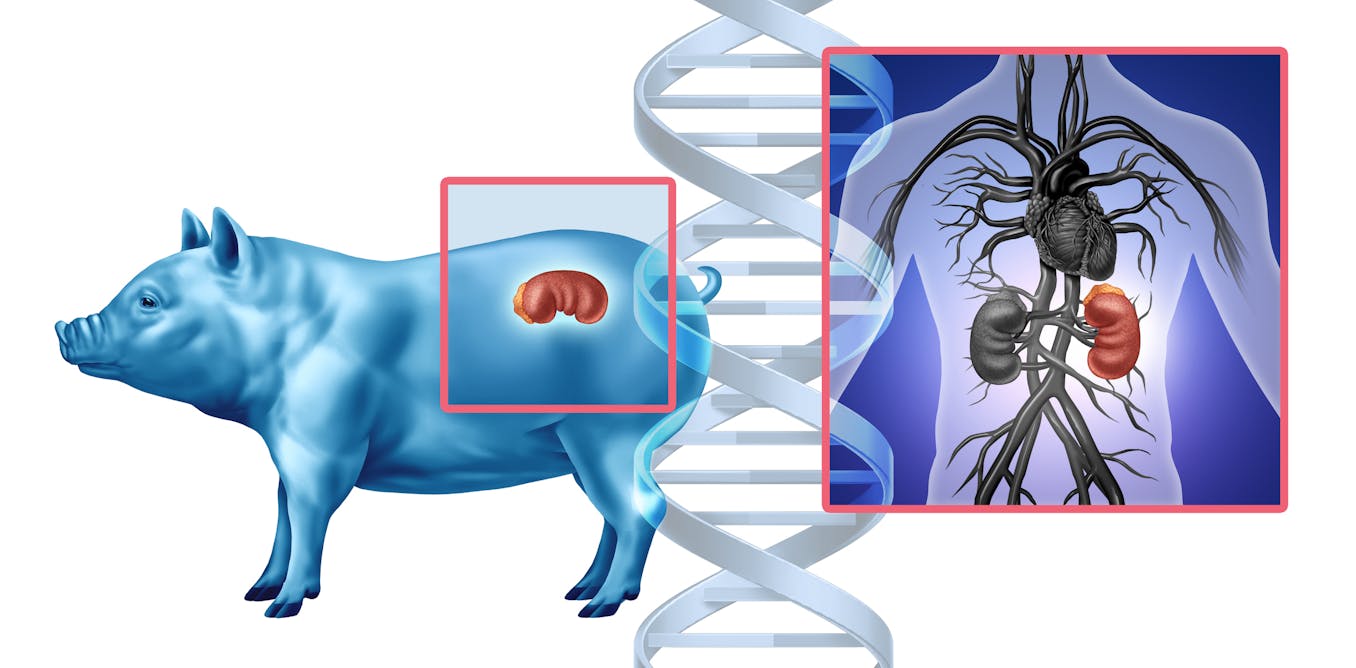
In a Maryland operating room one day in November 2025, doctors made medical history by transplanting a genetically modified pig kidney into a living patient. The kidney had been engineered to mimic human tissue and was grown in a pig, as an…