Says kites to be sold through authorised stalls, unsafe materials banned; free public transport arranged for festival
Punjab Minister for Information and Culture Azma Bukhari. Photo: File

Says kites to be sold through authorised stalls, unsafe materials banned; free public transport arranged for festival
Punjab Minister for Information and Culture Azma Bukhari. Photo: File
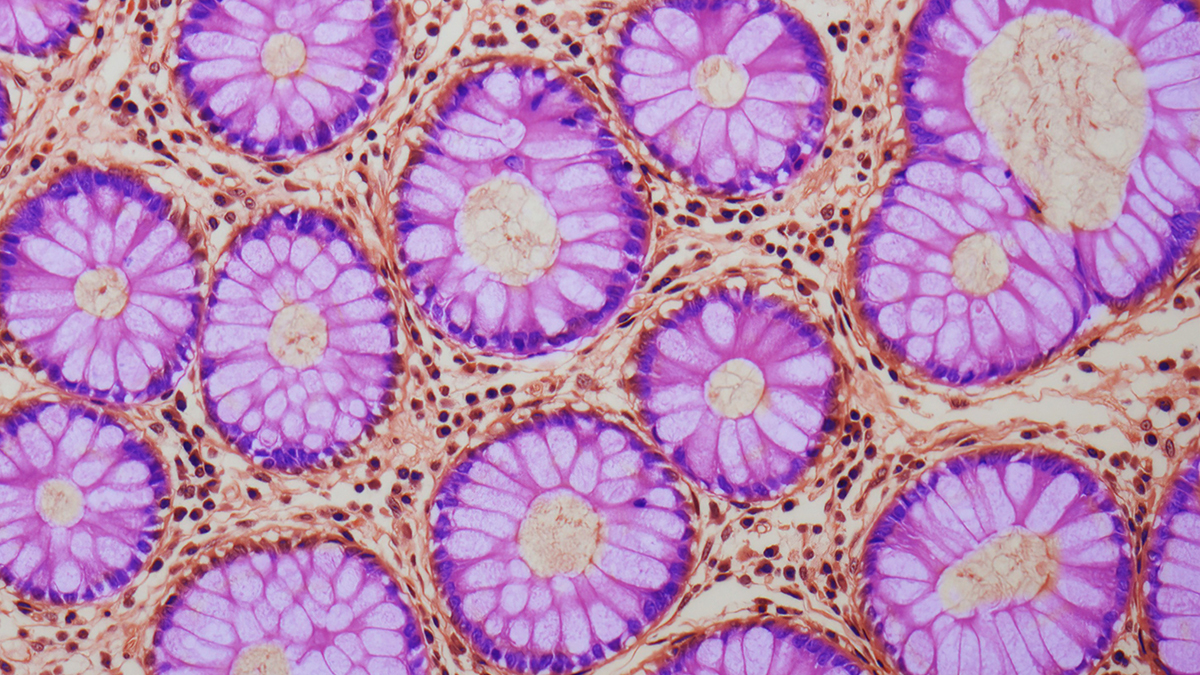
As we get older, chemical marks on our DNA slowly shift. Now, a study reveals this ‘drift’ in gut stem cells is fueled by inflammation and disrupted cell signaling, and it may help explain why our risk of colorectal cancer rises with…

Descend into the gloomy world of Garranox with Kill Team: Shadowhunt, the next expansion for the game of tactical skirmishes. It contains two new kill teams: the jump pack-equipped Murderwing, who revel in terror and slaughter, and the Celestian…

Defence minister voices support for creation of new provinces, says there is no harm in move and no one should fear it
Defence Minister Khawaja Asif speaking during a session at the Think Fest in Lahore on Saturday. Photo: Screengrab

Chinese automaker Changan has made headlines after its 4th-generation CS75 Plus gasoline-powered SUV set a Guinness World Record for the longest jump on an ice ramp, soaring 68.6 feet in an extreme cold environment of -22°F.
The CS75 Plus…


England captain Harry Brook criticised the pitch used for the second one-day international against Sri Lanka in Colombo, calling it “probably the worst” he had ever played on.
Brook’s side wrapped up a five-wicket victory over Sri Lanka thanks to…