Whether you’re a runner, cyclist, walker, love gym workouts, or just like to get your steps in, these premium watches can help you set goals and actively improve your wellbeing.
Both released in autumn 2025, the Garmin Venu 4 and Google Pixel…

Whether you’re a runner, cyclist, walker, love gym workouts, or just like to get your steps in, these premium watches can help you set goals and actively improve your wellbeing.
Both released in autumn 2025, the Garmin Venu 4 and Google Pixel…

The oldest known crocodile eggshells ever identified in Australia are giving UNSW researchers fresh insight into long vanished animals and the environments they depended on. These remains come from creatures that lived millions of years before…
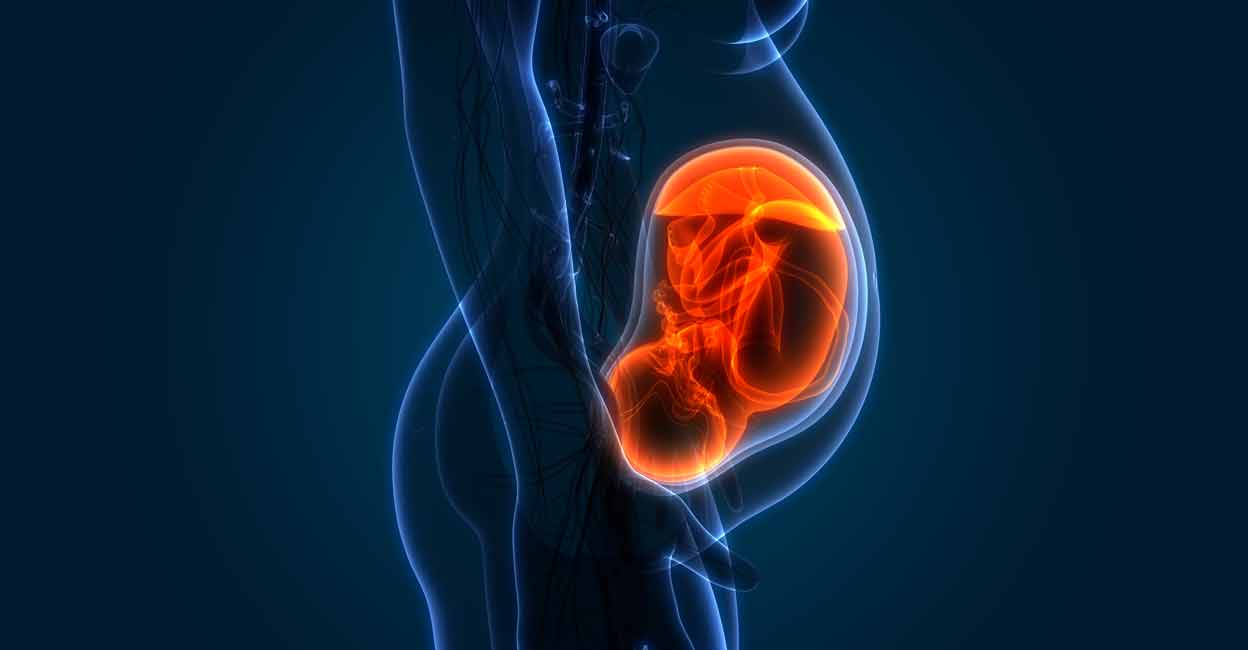
Women on an IVF journey or other infertility treatments often spend a lot of time wondering the how, when and why of getting pregnant. It’s not easy to deal with their bodies that play hard to get, especially while on multiple hormone…
Short-read sequencing has helped scientists identify and study genetic variants that increase Alzheimer’s disease risk, for example in the APOE, TREM2, and other genes. Yet these methods fail to capture the…

India’s campaign at the Kumamoto Masters Japan 2025 badminton tournament ended with Lakshya Sen’s semi-final defeat against local favourite Kenta Nishimoto on Saturday.
Competing at the Kumamoto Prefectural Gymnasium, Lakshya Sen, 15th in the…