- Match report: Champions League, FC Bayern v Union Saint-Gilloise FC Bayern
- Champions League: Harry Kane scores twice as Bayern move into last 16 BBC
- Highlights of UEFA Champions League matches on Jan. 21 Xinhua
- Kompany: ‘The team reacted well…
Author: admin
-
Match report: Champions League, FC Bayern v Union Saint-Gilloise – FC Bayern
-

AheadComputing Inc. Raises Additional $30M Seed2 Round to Reimagine CPU Architecture
The company will use the follow-on seed to advance the development of its high-performance CPU microarchitecture designed for the surging compute demands of AI workloads
PORTLAND, Ore., Jan. 22, 2026…
Continue Reading
-

UAE outlines approach to AI governance amid regulation debate at World Economic Forum
Media watchdogs condemn Israeli airstrike that killed 3 journalists in Gaza, call for investigation
DUBAI: Media watchdogs including the International Press Institute, the…Continue Reading
-
Champions League round-up: Barcelona win Slavia Praha thriller, Liverpool triumph over Marseille – UEFA.com
- Champions League round-up: Barcelona win Slavia Praha thriller, Liverpool triumph over Marseille UEFA.com
- Slavia Prague 2-4 Barcelona: Fermin Lopez double helps Barca win in Champions League BBC
- Live Blog! Slavia Prague vs Barcelona in the UEFA…
Continue Reading
-
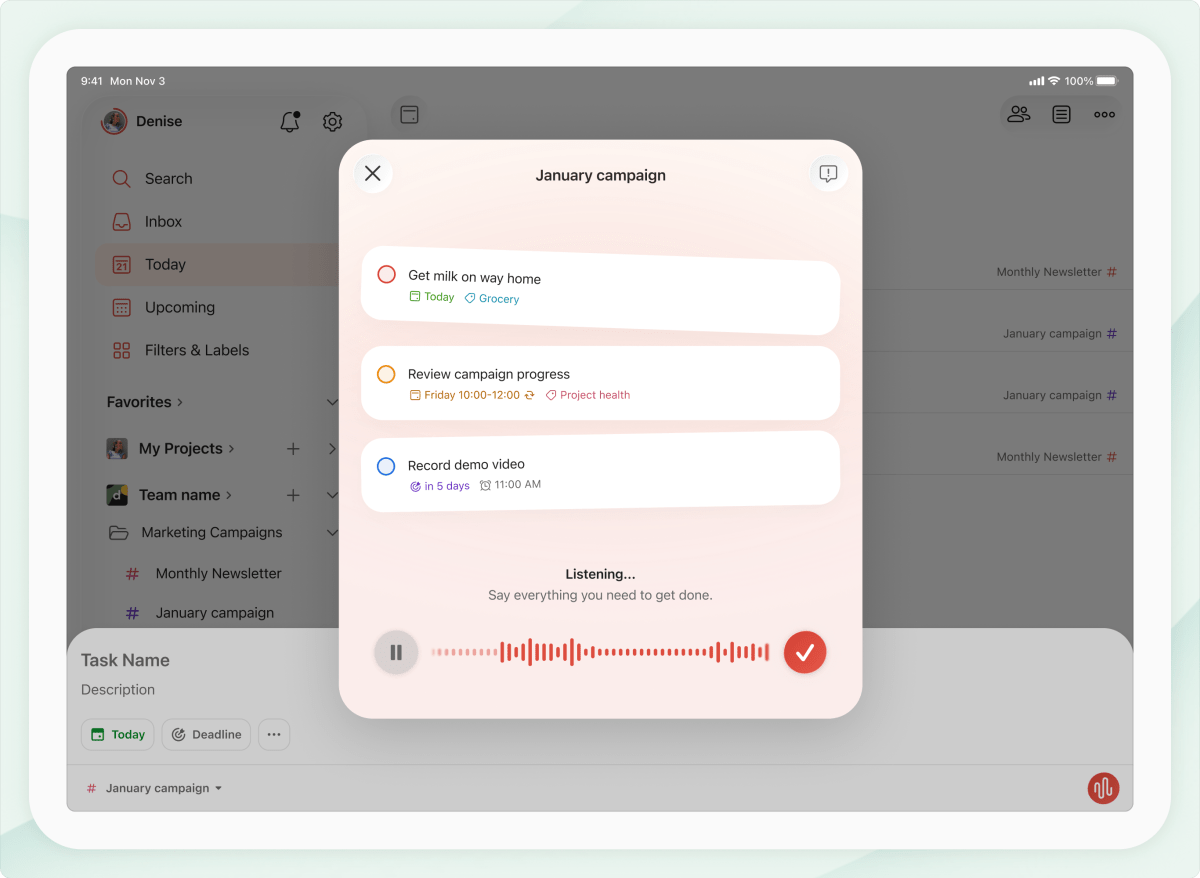
Todoist’s app now lets you add tasks to your to-do list by speaking to its AI
Maybe you don’t need an AI smart ring to keep up with your to-dos. Doist, the company behind Todoist, a popular to-do list app that works across multiple devices, is launching its new AI-powered, “voice-to-tasks” feature: Todoist Ramble….
Continue Reading
-

Marseille 0-3 Liverpool (Jan 21, 2026) Game Analysis
Mohamed Salah’s Liverpool return was less impactful than he would have hoped as he was overshadowed by Dominik Szoboszlai in a 3-0 victory in Marseille that made a Champions League top-eight finish all the more likely.
While all eyes were on the
Continue Reading
-

Chelsea 1-0 Pafos (Jan 21, 2026) Game Analysis
Chelsea will go into the final Champions League matchday in an automatic qualification spot after Moises Caicedo’s late header earned a nervy 1-0 win against Pafos at Stamford Bridge.
For a long time, head coach Liam Rosenior’s first European game…
Continue Reading
-

Barcelona player ratings vs Slavia Prague: Fermin Lopez bags brace as Robert Lewandowski atones for own goal but Pedri injury casts shadow over Champions League thriller
Barcelona recovered from a nightmare start to beat Slavia Prague 4-2 in the Champions League on Wednesday night. Fermin Lopez scored twice in the first half, while Dani Olmo and Robert Lewandowski were also on target for Hansi Flick’s side to…
Continue Reading
-

Molly Tuttle Partners with Martin Guitar to Launch Two New Signature Models at NAMM 2026
Drawing from her beloved 1943 D-18, the new D-18 Molly Tuttle & D-X2E Molly Tuttle embody the GRAMMY® winner’s sound & style.
NAZARETH, Pa., Jan. 21, 2026 /PRNewswire/ — C. F….
Continue Reading