Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…

Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…

A couple of weeks ago, Adam Mosseri posted to his grid. In a series of messages, Instagram’s top exec laid out his concerns for the platform in the coming year, largely around AI. The post was equal parts Working Through It, a sounding of…

Aurangzeb reviews economic reform agenda with ADB president; FM Dar inaugurates Pakistan pavilion
A meeting with Prime Minister Shehbaz Sharif and IMF Managing Director Kristalina Georgieva at the World Economic Forum meeting in Davos, Switzerland…

TOKYO — The world’s largest nuclear power plant restarted Wednesday in north-central Japan for the first time since the 2011 Fukushima nuclear meltdown, as resource-poor Japan accelerates atomic power use to meet soaring electricity needs.
The…

Gen X households now hold the most property wealth of any generation, as baby boomers downsize their homes and move more of their money into cash and retirement accounts.
Once known as the “slacker generation”, those born between 1965 and 1980 are mostly now aged over 50 years and have enjoyed years of inflated home prices.
Gen X households average $1.455m in wealth from dwellings and land, according to an analysis of ABS and census data by KPMG.
Sign up: AU Breaking News email
This compared to $1.36m in average property wealth among boomers – who remain the wealthiest overall, due to their super holdings and relative lack of debts.
Terry Rawnsley, an urban economist at KPMG, said the figures showed a generational “passing of the baton” when it came to property riches, which remains a “cornerstone” of Australians’ wealth.
In contrast, household property wealth among millennials – aged between 29 and 44 – was $890,000 on average, the analysis shows, reflecting lower rates of home ownership among this generation.
But with housing more unaffordable than ever, home ownership among younger households has dropped sharply and risks a major rise in intergenerational inequity, Rawnsley said.
By age bracket, households of those aged between 25 and 34 had $575,000 in property wealth on average – although this is offset by an average $346,000 in debt.
Rawnsley said while home ownership rates among older generations hovered about 80%, only around half of households in this younger cohort owned homes.
“The median house price at almost $1m across the country, so with that 50% home ownership rate you get to $500,000 in property wealth pretty easily”.
It was the remaining half of younger households who risk being left behind by the time they are in their 40s and 50s if they are unable to get on to the property ladder.
“If you miss out on that property purchase when you are in your 20s or 30s, the impact of that on your wealth will carry on for the next 30-40 years,” Rawnsley said.
With only slightly more than one in 10 homes for sale affordable for the average first home buyer, according to KPMG research, the pressure on younger Australians to make the right decisions was growing.
“There are a lot more difficult questions being asked of a 25-year-old today than there were 10, 20 or 40 years ago,” Rawnsley said.
“Being a ‘forever renter’ is not just about a lifestyle choice, it can be about locking in generational disadvantage – and not just for you, but for your kids as well, given the growing role of the bank of mum and dad.”
There has been strong criticism of the government’s first home buyer scheme on the basis it simply adds to demand, and therefore prices.
But Rawnsley’s “contrarian” view was that paying what he judged as 1-2% more for a home because of such schemes was worth it.
“If someone can save five years of rent in Sydney and get into the property market five years earlier, then that means a lot of people who can’t rely on the bank of mum and dad will ultimately be better off.”
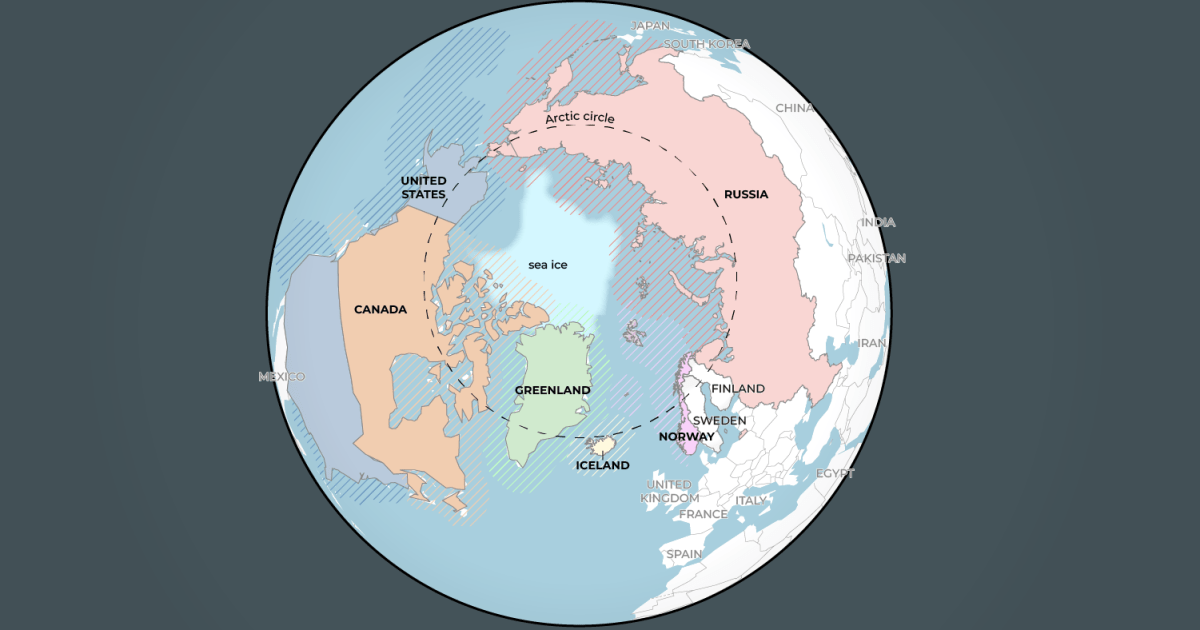
US President Donald Trump is in Davos, Switzerland, to attend the annual gathering of the World Economic Forum (WEF), where the issue of Greenland will be front and centre.
Trump’s long-running fixation on acquiring Greenland, an autonomous…

The two Tests, at Ellis Park (Saturday, 4 July) and Loftus Versfeld (Saturday, 11 July), will form part of double headers when the Boks host England in Johannesburg and Scotland in Pretoria.
The Bok Women and…