As a group, all TM cells share various molecular properties that distinguish them from other ocular cells. Our analyses determine the expression of IOP and glaucoma genes identified by GWAS in TM cell subtypes as well as in other limbal…
Author: admin
-

Psychometric Validation of the Chinese Version of the Diabetes Health
Introduction
Background
Between 1990 and 2022, the global prevalence of diabetes among adults (including both type 1 and 2) doubled, rising sharply from approximately 7% to about 14%, with the most significant increase occurring in low- and…
Continue Reading
-

Rare snow leopard spotted after 21 years in Chitral dies of natural causes
‘Its natural death indicates the species still thrives in Chitral valleys,’ says Chitral divisional forest officer
Snow leopard in Chitral dies of natural causes. PHOTO: EXPRESS
…Continue Reading
-

A Reading List by Ocean Vuong: Part Two
Lead ImageTommy Kha, Headtown (XII), Midtown Memphis, 2021Courtesy of Tommy Kha and Higher Pictures
Continue Reading
-

ISU Figure Skating Four Continents Championships 2026: All results, scores and standings
ISU Four Continents Championships 2026: Full schedule
All times China Standard Time (UTC+8)
Thursday, 22 January
- 12:15: Women – Short program
- 16:30: Ice Dance – Rhythm dance
- 20:00: Pairs – Short program
Friday, 23 January
- 12:00: Ice…
Continue Reading
-

Extended opening hours for Aberdeen pubs
Hospitality businesses in Aberdeen will be allowed extended opening hours for Scotland’s matches during the World Cup this summer.
The national team will face Haiti in their first fixture at the tournament for 28 years on 14 June, with kick off at…
Continue Reading
-

Geonordic expands Nordic offering with Tersus GNSS receiver agreement
Finnish geotechnical solutions provider Geonordic has signed a new cooperation agreement with Tersus GNSS, granting Geonordic exclusive resale rights for the Luka GNSS receiver in Finland and Sweden. The…
Continue Reading
-
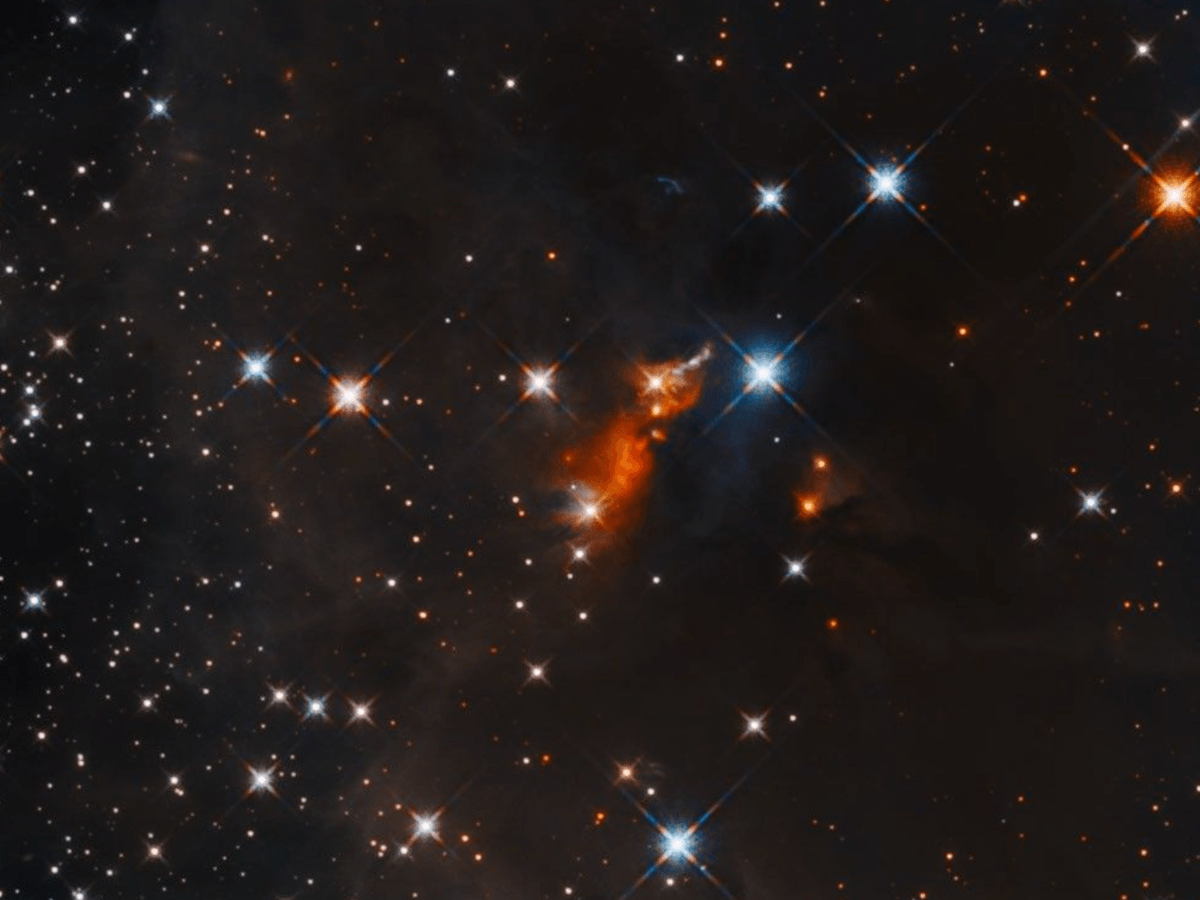
NASA’s Hubble Telescope captures striking images of developing stars
The Hubble Telescope took snapshots of the infant stars, in an effort to understand how larger stars are formed.
As part of the Sofia Massive Star Formation Survey, which is investigating how massive stars,…
Continue Reading
-

Islamic State group claims blast on Chinese restaurant in Kabul that killed 7
KABUL, Afghanistan — The Islamic State group has claimed responsibility for an explosion at a Chinese restaurant in Afghanistan’s capital that killed at least seven people, including a Chinese national, and wounded more than a dozen others.
In…
Continue Reading
-

Boeing and Ethiopian Airlines Announce Order for Nine 787 Dreamliners
- East African airline will leverage more 787-9 jets to expand international network
- Ethiopian also completes purchase of 11 more 737 MAX jets
ADDIS ABABA, Ethiopia, Jan. 20, 2026 /PRNewswire/ — Boeing (NYSE: BA) and Ethiopian Airlines announced today Africa’s largest carrier ordered nine 787 Dreamliner airplanes as demand for long-haul travel continues to rise. Ethiopian Airlines will leverage the 787-9 jets to grow its route network, which currently serves 145 international destinations.
The airline’s latest 787 purchase follows its commitment for 11 737 MAX jets announced at the Dubai Airshow. Both orders were finalized in December 2025 and boosts Ethiopian Airlines’ order book by a total of 20 fuel-efficient Boeing airplanes.
“We are pleased to confirm the order for nine Boeing 787 Dreamliner aircraft to further expand our existing fleet. This order underscores our continued commitment to enhancing our fleet with modern, fuel-efficient aircraft, thereby further strengthening our customer service,” said Mesfin Tasew, Ethiopian Airlines Group CEO. “We will continue to acquire more aircraft and adopt the latest technologies as part of our strategic vision to advance sustainable aviation.”
Ethiopian Airlines operates Africa’s largest 787 Dreamliner fleet, flying its 787-8 and 787-9 jets on intercontinental routes from Addis Ababa to high-demand destinations across Europe, Asia and North America as well as key intra-African routes spanning the world’s second-largest continent.
“The 787 Dreamliner family has proven to be a game-changer for airlines around the world, and we are proud to support Ethiopian Airlines in their mission to connect Africa with the global community,” said Anbessie Yitbarek, Boeing vice president of Commercial Sales and Marketing for Africa. “Together, we look forward to shaping the future of air travel with advanced, efficient and comfortable airplanes to serve their passengers.”
The capacity and efficiency of the 787 Dreamliner, which reduces fuel use and emissions by 25% compared to the airplanes it replaces, enables Ethiopian Airlines to transport passengers point-to-point across Africa while accommodating cargo in the belly of the airplane for high-demand trade lanes.
Since 2011, the 787 Dreamliner family has helped airlines open more than 520 new nonstop routes between city pairs that were never previously served and carried more than 1 billion passengers.
Ethiopian Airlines operates the largest Boeing airplane fleet in Africa and has the continent’s largest backlog of 737 MAXs, 777X and 787 Dreamliner airplanes.
A leading global aerospace company and top U.S. exporter, Boeing develops, manufactures and services commercial airplanes, defense products and space systems for customers in more than 150 countries. Our U.S. and global workforce and supplier base drive innovation, economic opportunity, sustainability and community impact. Boeing is committed to fostering a culture based on our core values of safety, quality and integrity.
Contact
Boeing Media Relations
media@boeing.comSOURCE Boeing
Continue Reading
