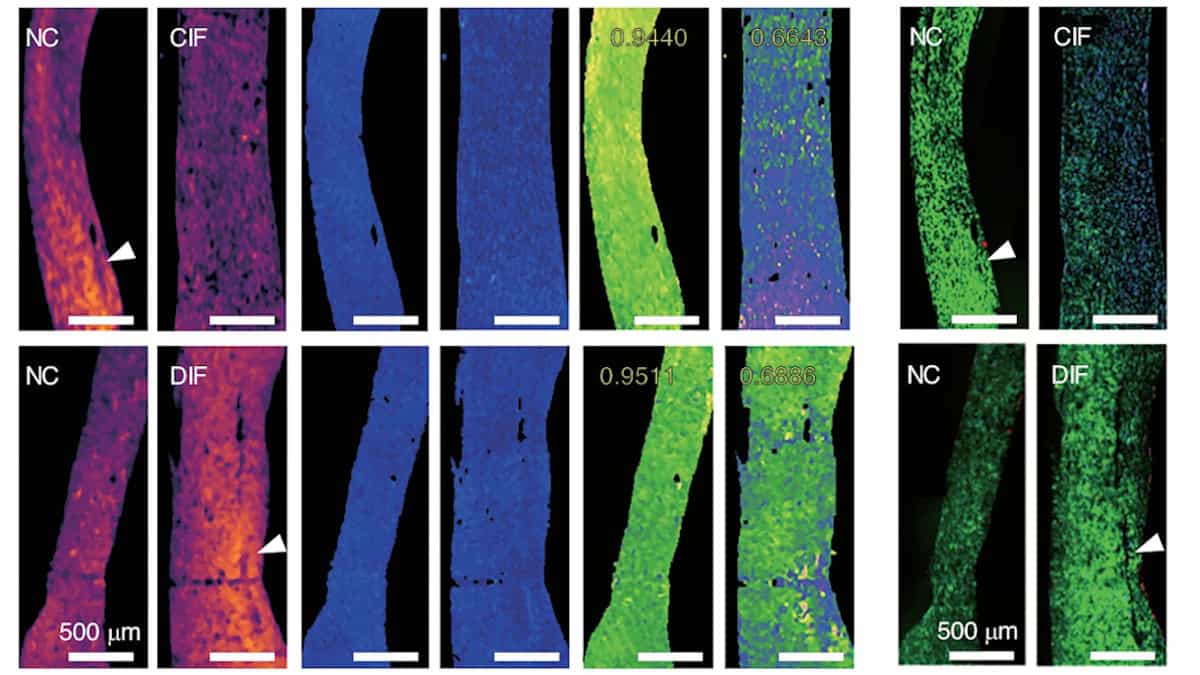
A new discovery about how cells communicate with each other in the body’s immune system has revealed deeper insights for an international team of scientists into fundamental immune system function.
The new study, in Nature…
A new discovery about how cells communicate with each other in the body’s immune system has revealed deeper insights for an international team of scientists into fundamental immune system function.
The new study, in Nature…

Even outside hi-fi circles, the release of a new AirPods Pro is a pretty monumental occasion.
Which is why we’re so glad the latest model lives up to the hype: we awarded the buds fives stars only days ago, in our AirPods Pro 3 review.
Almost…
Foldable smartphones have become a defining feature of modern technology, combining compact designs with expansive, flexible displays. However, a persistent issue has limited their practicality: the use of glued-in batteries. These…

Astronomers have revealed the James Webb Space Telescope’s (JWST) sharpest-ever image of the area around a black hole. The spectacular view could help solve a decades-long mystery while reversing a long-held belief about space’s most extreme…

While the suit proved an important introductory moment, immediately—and wordlessly—letting the audience know where Harper landed after striking a deal with Otto at the end of Season 3, Smith wanted to ensure that the wunderkind banker lived…