- US, Pakistan agree to strengthen security and border cooperation Arab News
- Pakistan, US agree to enhance collaboration in security, border management Dawn
- US, Pakistani troops conclude joint training exercise ‘Inspired Gambit 2026’ in Pabbi The…
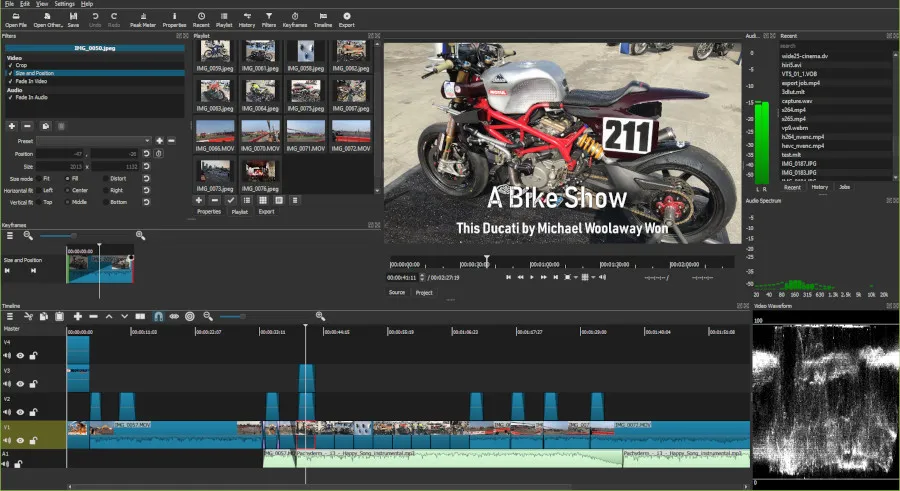
The Shotcut 26.1 beta was released overnight as the newest version of this Qt6-based, cross-platform video editing solution. Standing out the most with this new development release are some new GPU-accelerated hardware decode options for aiming…

Michelle Randolph has finally broken her silence on continuous chatters about her romance with Glen Powell.
The couple first sparked…

NEW DELHI: India captain Ayush Mhatre and Bangladesh vice-captain Zawad Abrar skipped the customary handshake at the toss ahead of their Group A clash in the ICC Under-19 World Cup at the Queens Sports Club on Saturday, underlining the strain…
MELBOURNE, Jan. 17 (Xinhua) — Jannik Sinner will be aiming for a third straight men’s title, while top women’s seed Aryna Sabalenka strives to reclaim her crown at the Australian Open, where China will be well represented at the…

The moon moves in front of the sun in a rare “ring of fire” solar eclipse as seen from Singapore on December 26, 2019. (Photo by Louis KWOK / AFP) (Photo by LOUIS KWOK/AFP via Getty Images)
AFP via Getty Images
One month from today, on Tuesday,…

Angela FergusonNorth West
 Getty Images
Getty ImagesA proposed in-hospital cinema in Manchester would bring a touch of “magic” to the lives of patients and their families, Rachael and…