Key events
Protests planned in Denmark, Greenland against Trump’s calls for annexation
Hello and welcome to our live coverage of today’s events.
A series of demonstrations have been…

Key events
Hello and welcome to our live coverage of today’s events.
A series of demonstrations have been…

The Islamabad High Court (IHC) on Saturday ordered the Capital Development Authority (CDA) not to cut trees until February 2. Issuing a four-page order, Justice Khadim Hussain Soomro also issued notices to the CDA, Ministry for…
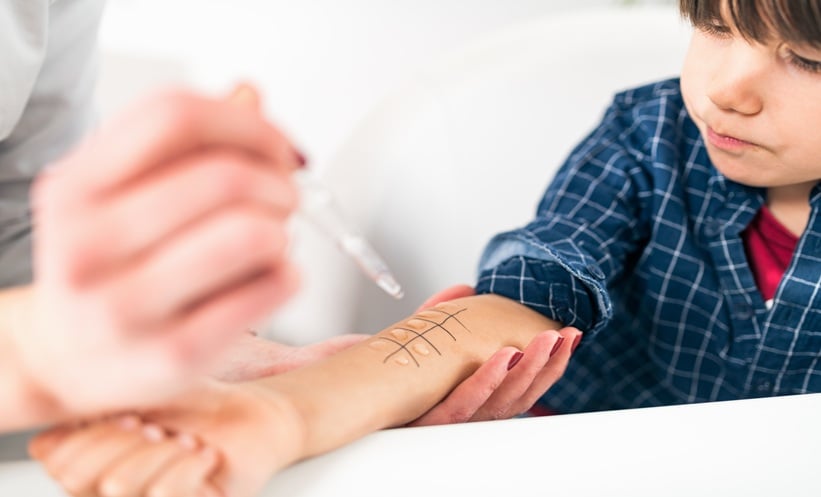
EARLY colonisation of the infant gut by specific Bifidobacterium strains was linked to a significantly reduced allergy risk in childhood, according to new research that highlighted the importance of microbial exposure and feeding…

The main draw for the Australian Open 2026, the first Grand Slam tennis tournament of the year, will begin at Melbourne Park on Sunday.
The 114th edition of the Australian Open tennis tournament will be available for live streaming and telecast…

Chocolate eggs are looking smaller than ever this year and it is not just because Easter is still so far away.
Many of the Easter eggs already out on supermarket shelves this month not only cost more, but have been reduced in size or weight as the price of cocoa has driven a new wave of shrinkflation.
Maltesers is living up to its “the lighter way to enjoy chocolate” slogan with its XL egg rather less large this year at 194g in many shops, down from 231g in 2025, while the price charged by Tesco has risen by £1 to £7.
The weight loss is largely down to there being one fewer mini pack of Maltesers inside the box, according to trade journal the Grocer, meaning the price per gram is up 39% to 3.6p.
A similar move with Cadbury’s Twirl eggs – which now include only two individually wrapped Twirl fingers rather than two full bars – means they have shrunk by 9.5% or 23g to 218g. However, the price in most shops has risen to £7 from £6 last year, leading to a price per gram increase of more than 28%.
Four fewer eggs in a Mini Eggs family pack means the Cadbury’s treat is now 4% smaller at 256g compared with 270g a year ago, while the price has risen to £6.20 at Tesco for those without a loyalty card, compared with £4.85 a year ago. That is equivalent to a 35% increase in price – although the same pack is priced at £5.50 at Sainsbury’s and Waitrose and £4.94 at Asda for those who wish to shop around.
Lindt gold bunnies have held their ground at 200g for the largest option, but are now £8.50 at most retailers (without a loyalty card), a £3 jump from their £5.50 price at Tesco a year ago.
The cost of chocolate confectionery has increased sharply in the past few years as cocoa prices have soared after poor harvests in the main growing regions of Ghana and Côte d’Ivoire over the past three years, amid extreme temperatures and unusual rainfall patterns driven by the climate crisis.
With sugar, energy and labour costs also on the rise, manufacturers have turned to a variety of tactics, from making bars and biscuits smaller to reducing cocoa content in an effort to keep the prices paid by shoppers down.
In October, McVitie’s reduced the amount of cocoa in the recipes of Club and Penguin bars so much they are now only “chocolate flavour”.
A spokesperson for the Maltesers owner Mars Wrigley’s UK and Ireland business said: “We understand the cost pressures that shoppers continue to face and always aim to absorb rising costs wherever possible.
“However, ongoing pressures, driven in part by well-documented rises in the cost of cocoa, mean we have had to make carefully considered changes. Decisions around product size are not taken lightly, but they help to ensure shoppers can continue to enjoy their favourite Easter treats without any compromise on the quality or taste they expect from Mars. As with all our products, final pricing remains at the discretion of individual retailers.”
A spokesperson for Cadbury’s owner Mondelēz International said retailers were free to set their own prices, adding: “We understand the economic pressures that consumers continue to face and any changes to our product sizes is a last resort for our business.
“However, as a food producer, we are continuing to experience significantly higher input costs across our supply chain, with ingredients such as cocoa and dairy, which are widely used in our products, costing far more than they have done previously.
“Meanwhile, other costs like energy and transport, also remain high. This means that our products continue to be much more expensive to make and while we have absorbed these costs where possible, we still face considerable challenges.
“As a result, we are having to make carefully considered changes to the recommended promotional price alongside small weight reductions to our Cadbury Twirl Easter Egg (218g), Cadbury Wispa Easter Egg (177g) and Cadbury Mini Egg bags (256g).”
Lindt was approached for comment. Tesco declined to comment.

The best all-in-one portable projector is the one that makes all the right compromises. It needs to balance image and sound quality with battery life and responsiveness in a device that’s not too expensive and small enough to take anywhere.

The Strikers finished their BBL|15 season in style, dismantling the Renegades in front of a roaring Adelaide Oval crowd.
The spin of Tabraiz Shamsi, Jerrssis Wadia and Lloyd Pope was too much for the Renegades as they…

Iran’s deadly crackdown appears to have broadly quelled protests for now, residents said on Friday, as state media reported more arrests in the shadow of repeated US threats to intervene if the killing continues.
Meanwhile, a senior…