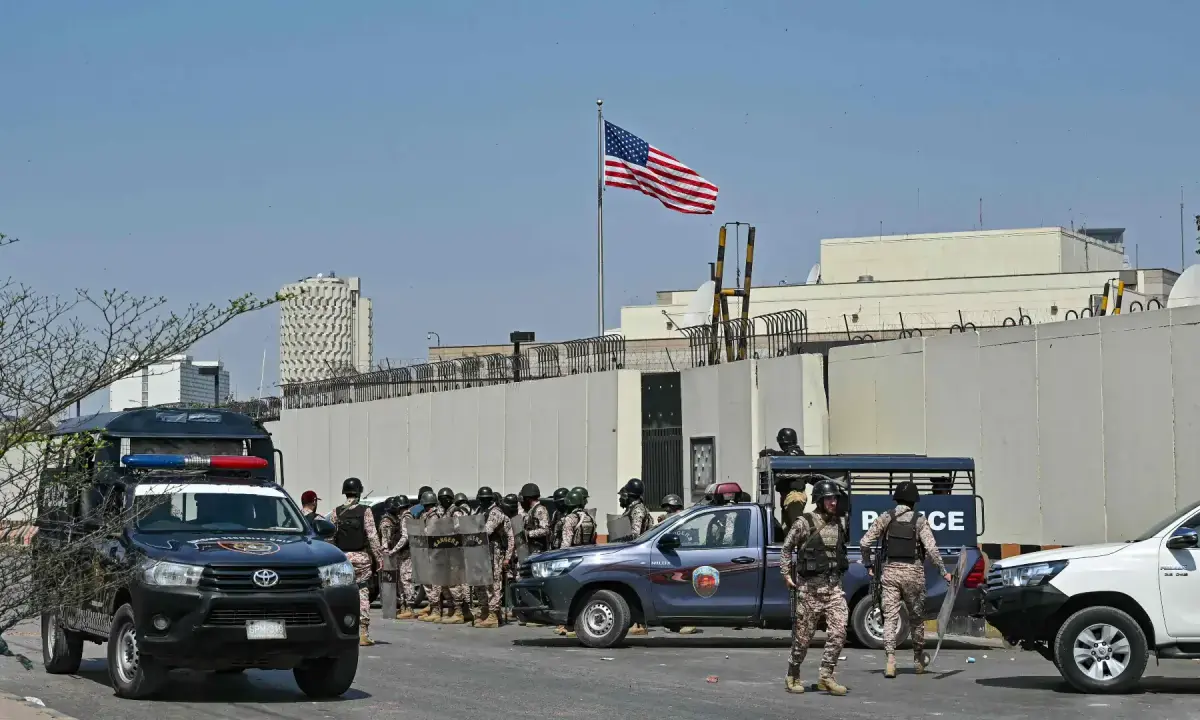
- War on Iran aimed at bringing Israeli influence to Pakistan’s border: Asif Business Recorder
- Asif warns Iran war could fuel Taliban-India-Israel nexus encircling Pakistan The Express Tribune
- ‘Zionist agenda’: Asif says war on Iran aims to bring…
Interim summary
In case you’re just joining us, here’s a snapshot of the key developments as the US-Israel war on Iran dramatically expands across the Middle East.
-
Israeli and US warplanes launched a fresh wave of strikes across Iran,…