“It was long rallies, very, very different rhythm and it was also about my focus today,” she said.
“I lost a bit of focus on the first set, dropped the set, but actually didn’t give up and was trying just to keep this positive attitude,…

“It was long rallies, very, very different rhythm and it was also about my focus today,” she said.
“I lost a bit of focus on the first set, dropped the set, but actually didn’t give up and was trying just to keep this positive attitude,…
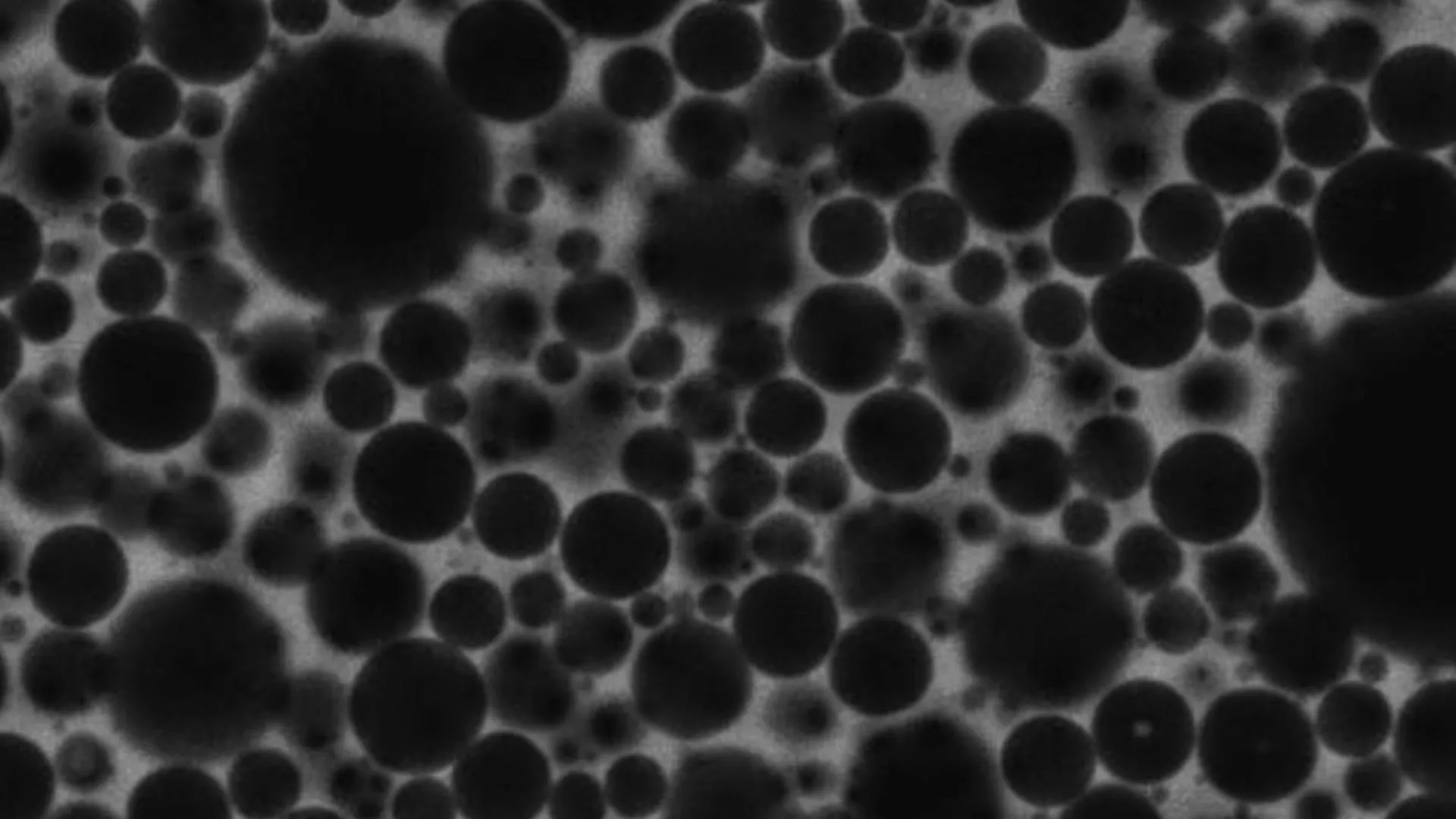
Foams appear in everyday life as soap suds, shaving cream, whipped toppings and food emulsions like mayonnaise. For many years, scientists believed foams behaved much like glass, with their tiny components locked into disordered but essentially…

Foams appear in everyday life as soap suds, shaving cream, whipped toppings and food emulsions like mayonnaise. For many years, scientists believed foams behaved much like glass, with their tiny components locked into disordered but essentially…
…
Several NATO countries are deploying small numbers of military personnel to Greenland to participate in joint exercises with Denmark as US President Donald Trump ramps up his threats to forcibly annex the Arctic…

If you like playing daily word games like Wordle, then Hurdle is a great game to add to your routine.
There are five rounds to the game. The first round sees you trying to guess the…
Over two weeks into wide-scale protests against the Islamic Republic regime in Iran, the death toll and number of arrests are rapidly mounting. Iranian human rights organizations place the number of dead at 2,500, while other sources suggest it…

It’s day 26 of the lunar cycle, so there’s not much to see on the Moon’s surface tonight. If you have viewing gear, however, you may still catch a glimpse. Keep reading to see what’s…

On January 13, at least four members of a peace committee were killed in a suspected terrorist attack in Pakistan’s northwestern Khyber Pakhtunkhwa province in Bannu district.
In Pakistan, peace committees are local groups that help…

Donald Trump has said he has been assured the killing of protesters in Iran has been halted, adding that he would “watch it and see” about threatened US military action, as tensions appeared to ease on Wednesday night.
Trump had repeatedly…