The Trump administration has indefinitely suspended immigrant visa processing for people from 75 countries, marking one of its most expansive efforts yet to restrict legal pathways to the United States.
The freeze, which takes effect on 21…

The Trump administration has indefinitely suspended immigrant visa processing for people from 75 countries, marking one of its most expansive efforts yet to restrict legal pathways to the United States.
The freeze, which takes effect on 21…
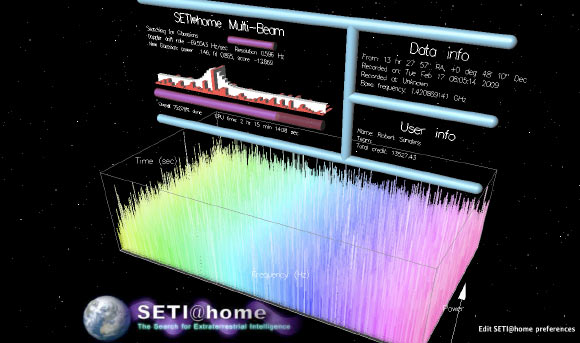
SETI@home, the pioneering distributed-computing project launched in 1999 that enlisted millions of volunteers to analyze radio signals from space, produced some 12 billion detections — brief bursts of energy that stood out from background…

“Landslide” by Stevie Nicks of Fleetwood Mac and “Purple Rain” by Prince find chart success after appearing in Stranger…
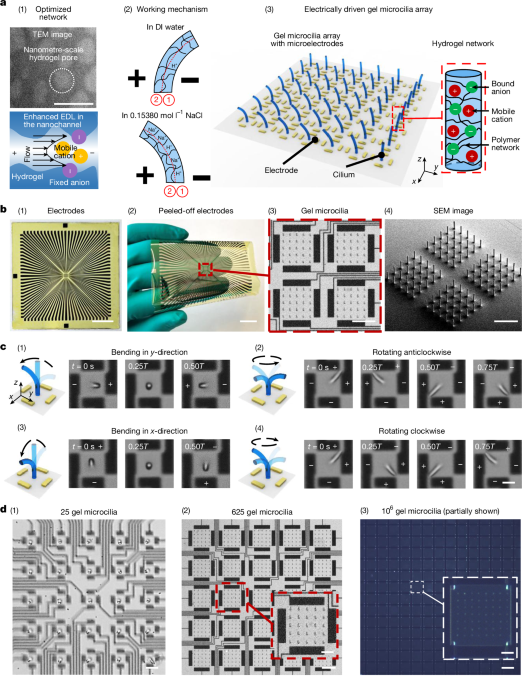
Extended Data Fig. 2 illustrates the fabrication process of single-layer microelectrodes. Below are the step-by-step details:
Step 1. Preparation of the polyimide substrate (Extended Data Fig. 2a). This…


.
Karachi Mayor Murtaza Wahab’s newly announced plan to dismantle the city’s notorious “tanker mafia” and phase out the city’s flailing hydrant-based…

The NBA introduced a new twist to the regular season in 2022 by dedicating a week in January to classic and emerging rivalries between teams and players. In 2026, the league will celebrate the fifth annual AWS NBA…

Khamenei, now 86, has ruled Iran as supreme leader since 1989 after succeeding Ayatollah Khomeini
Iran’s Supreme Leader Ayatollah Ali Khamenei looks on, in a televised message following the Israeli strikes in Tehran, Iran, June 13, 2025. Photo:…