Venus Williams, overcoming health issues on her comeback path
The 2025 campaign was as significant on the court as it was off it for Williams. She returned to the WTA Tour after a year of inactivity with a wildcard win in Washington D.C., before…

The 2025 campaign was as significant on the court as it was off it for Williams. She returned to the WTA Tour after a year of inactivity with a wildcard win in Washington D.C., before…

SUNNYVALE, Calif., Jan. 14, 2026 /PRNewswire/ — Covalent Metrology today announced it has rebranded as Covalent and launched a new website reflecting a broader scope of products…

The Trump administration is placing an indefinite pause on immigrant visa processing from 75 countries, further restricting the legal routes for entering the US.
The State Department on Wednesday said the administration wants to bring “an end to…

New designs will be introduced for banknotes of Rs100, Rs500, Rs1,000 and Rs5,000 denomination
The federal government has decided to introduce…

SpaceX added 29 more Starlink satellites to its low Earth orbit megaconstellation today (Jan. 14).
A Falcon 9 rocket carrying the broadband internet relay units launched from Space Launch Complex 40 at Cape Canaveral Space Force Station in…
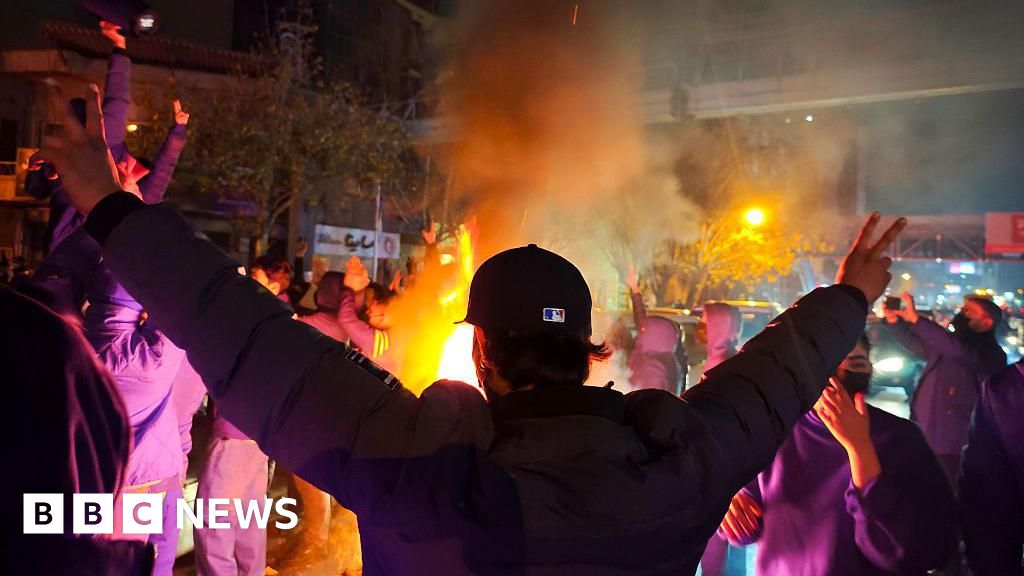
Reha Kansara and Ghoncheh HabibiazadBBC News and BBC Persian
 Getty Images
Getty ImagesStarlink has reportedly…

LONDON, Jan 13 (Reuters) – Dozens of commercial ships have dropped anchor at a distance outside Iran’s port limits in recent days, according to data and shipping sources, as tensions with the United States grow.
Such movements were precautionary…