- ‘It is bittersweet’: Crew-11 astronaut hands over control of ISS ahead of 1st-ever medical evacuation Space
- Astronaut’s ‘serious medical condition’ forces Nasa to end space station mission early Dawn
- ISS astronaut medical evacuation latest…
Author: admin
-
'It is bittersweet': Crew-11 astronaut hands over control of ISS ahead of 1st-ever medical evacuation – Space
-

No. 8 Huskers Look for 17th Win Tuesday against Oregon – University of Nebraska
No. 8 Huskers Look for 17th Win Tuesday against Oregon
The No. 8/10 (AP/Coaches) Nebraska men’s basketball team returns home Tuesday night, as the Huskers welcome Oregon to Lincoln. Tipoff between the Huskers and Ducks from a sold-out Pinnacle…Continue Reading
-

Amendment 34: A.6 Landslide Change Characterization Experiment Science Team Final Text and Due Dates
A.6 Earth Venture Suborbital-4 Landslide Change Characterization Experiment (LACCE) Science Team (ST) solicits proposals to build the full LACCE Science Team, including team members that will provide airborne and ground-based measurements,…
Continue Reading
-

All-State Band, Choir and Orchestra 2026
RISD Fine Arts proudly announces the selection of 20 RISD students to All-State band, choir or orchestra. These students were selected through a rigorous statewide audition process and will participate in the Texas Music Educators…
Continue Reading
-
Researchers uncover how E. coli bacteria sneak into the prostate – Medical Xpress
- Researchers uncover how E. coli bacteria sneak into the prostate Medical Xpress
- Uropathogenic Escherichia coli invade luminal prostate cells via FimH–PPAP receptor binding Nature
- Secret Route Of Prostate Infections Mirage News
- University of…
Continue Reading
-

Home Audio, Headphones, Turntables & More
All products and services featured are independently chosen by editors. However, Billboard may receive a commission on orders placed through its retail links, and the retailer may receive certain auditable data for accounting purposes.
The…
Continue Reading
-
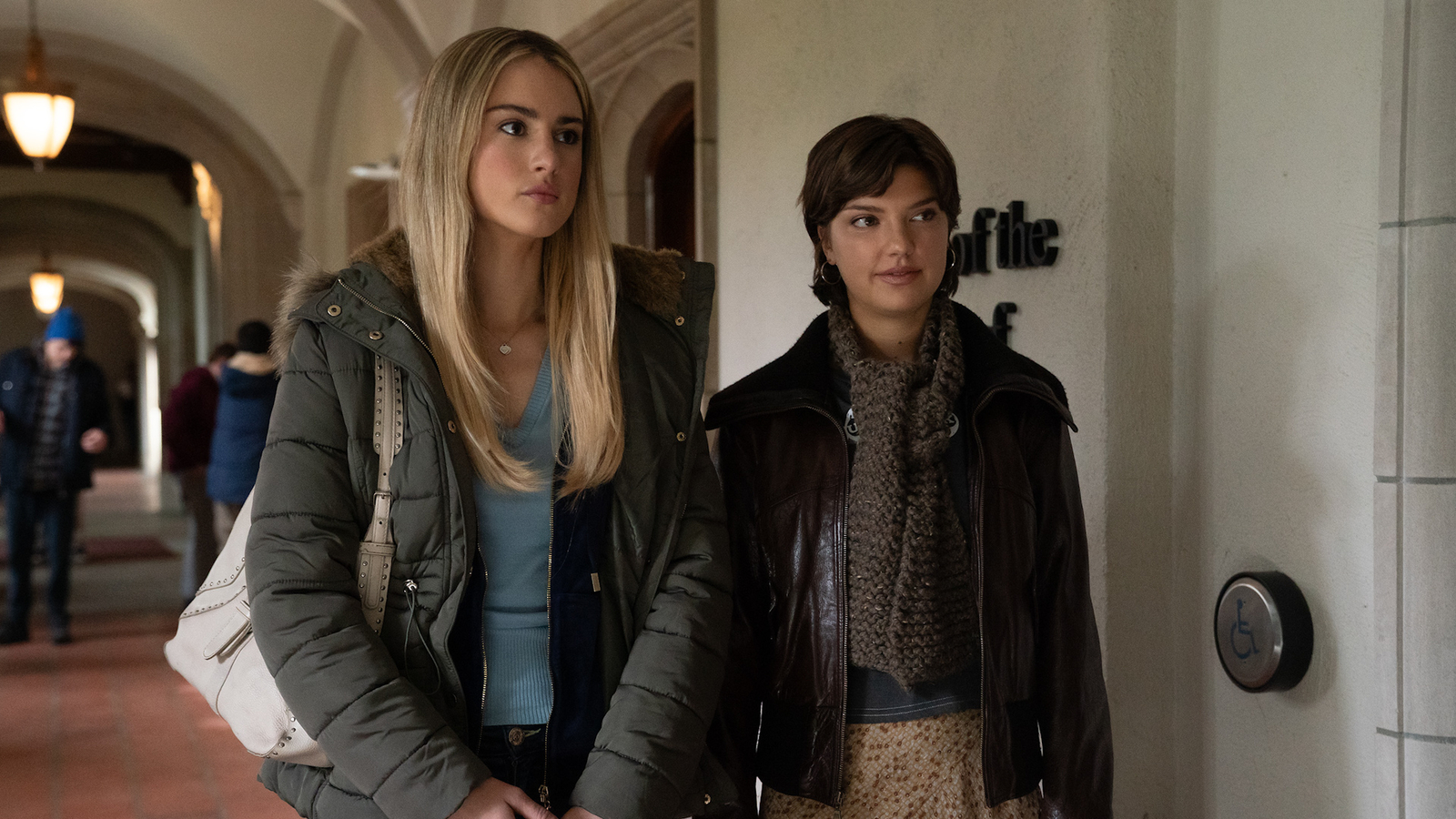
“Tell Me Lies” EP says fan reactions drove her to write a specific storyline for season three
Hold onto your hats, “Tell Me Lies” fans. You might not be prepared for everything that takes place in season three.
Warning: this article contains major season two spoilers.
Episode one picks up where we left off, in the 2015 timeline at Bree and…
Continue Reading
-

F.6 Science Activation Corrections and Other Documents posted
The Science Mission Directorate Science Activation Program encourages all people to actively participate in science through activities and resources developed by a collaborative network of project teams drawing on NASA SMD assets (science…
Continue Reading
-
NASA taps Colorado companies to lead development on telescope searching for life on other planet – Denver Gazette
- NASA taps Colorado companies to lead development on telescope searching for life on other planet Denver Gazette
- NASA seeks to accelerate development of Habitable Worlds Observatory SpaceNews
- Astroscale Unit Selected for NASA Study on Space…
Continue Reading
