Minister of State for Interior Talal Chaudhry strongly criticized Khyber Pakhtunkhwa Chief Minister Sohail Afridi by referring to the latter’s ‘vague remarks’ on terrorism.
Addressing a press conference, he accused…

Minister of State for Interior Talal Chaudhry strongly criticized Khyber Pakhtunkhwa Chief Minister Sohail Afridi by referring to the latter’s ‘vague remarks’ on terrorism.
Addressing a press conference, he accused…

The arrival of winter brings pleasant weather and cozy vibes, but it also comes with several health challenges.
Cold winds and low temperatures can increase the risk of colds, flu, and fatigue. However, with a few…

A mother-of-two with a brain tumour is preparing to raffle off her own family home to fund potentially life-saving cancer treatment in Germany.
Claire Nutter, from Burnley, was given the diagnosis in 2023 after she kept getting headaches and…

Zoe KleinmanTechnology editor
 BBC
BBCHere’s me, at the end of a pier in Dorset in the summer.
Two of…

I was three when I started. By the time I was five, I thought I was pretty well fully qualified. I’d seen it all. I’d seen a lot. We were doing 15 or 20 calls a day.
I remember very, very well suffering agonies of cold in my father’s cars….

After a three-and-a-half-month journey, NASA’s Interstellar Mapping and Acceleration Probe (IMAP) has finally reached its destination, a strategic location between the Sun and Earth where it will begin its groundbreaking mission to…
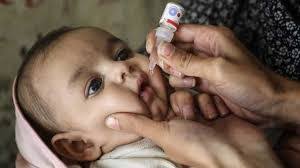
Deputy Commissioner Captain (R) Zulfiqar Ali Karar chaired a key meeting to ensure full polio vaccination coverage. Focus included field monitoring,…

CHARLOTTESVILLE, Va. – No. 16 Virginia (14-2, 3-1 ACC) travels to No. 20 Louisville (12-4, 2-2 ACC) on Tuesday, Jan. 13. Tipoff for the ACC contest at KFC Yum! Center is set for 7 p.m. on ESPN2 and Virginia Sports Radio Network.
For…

January 12, 2026 — The Walton High School (WHS) Sports Hall of Fame (HOF) recently introduced its 2026 class of honorees. Former Major League Baseball (MLB) pitcher Marc Pisciotta and National Football League (NFL) draftee Mike Travis were…