Construction to replace the dock on Route 638 (Jones Creek Road) will begin in July 2026 with anticipated completion in late September 2026
Last updated: January 9, 2026
Construction to replace the dock on Route 638 (Jones Creek Road) will begin in July 2026 with anticipated completion in late September 2026
Last updated: January 9, 2026
The environment has never been more favorable for existing banking organizations launching a digital asset business and those Fintech and other nonbank companies considering acquiring or chartering a full-service or limited-purpose bank in order to operate a digital asset business. At the end of 2025, U.S. banking regulators continued to take major steps signaling a considerably more open supervisory posture regarding digital asset activities.
First, in December, the Office of the Comptroller of the Currency (OCC) conditionally approved five applications to either newly charter or convert existing institutions into national trust banks that will engage in digital asset activities. Sidley represented FMR LLC, the parent company of Fidelity Investments, in obtaining an approval permitting the conversion of its New York limited purpose trust company into a national trust bank. These approvals built upon a series of interpretive letters released by the OCC in 2025 expanding the range of permissible digital asset activities for national banks as well as the OCC’s rescission of an earlier interpretive letter that required formal nonobjection from the regulator before a national bank or federal savings association could engage in certain digital asset activities. Finally, on January 8, the OCC issued a proposed rulemaking that would clarify, but not change the scope, of its authority to charter national trust banks.
Second, the Board of Governors of the Federal Reserve System (the Federal Reserve) (i) rescinded and replaced a 2023 policy statement regarding state member bank novel activities with a new, more permissive policy statement and (ii) published a request for information seeking public comment on a proposed special purpose Federal Reserve Bank “payment account” prototype (informally, a “skinny master account”) aimed at institutions focused on payments innovation.
Finally, the U.S. Department of the Treasury (Treasury) published an advance notice of proposed rulemaking (ANPRM) seeking public comment relating to the implementation of the Guiding and Establishing National Innovation for U.S. Stablecoins Act (GENIUS Act), while the Federal Deposit Insurance Corporation (FDIC) issued a notice of proposed rulemaking (NPRM) establishing an application process for subsidiaries of FDIC-supervised insured depository institutions to issue payment stablecoins under the GENIUS Act.
OCC Conditional Approvals for National Trust Bank Charters
On December 12, 2025, the OCC announced the conditional approval of five national trust bank charter applications from institutions proposing to offer digital asset products and services. Of the five applications, two were for de novo national trust bank charters, while three were for conversions from a state trust company to a national trust bank. National trust banks engage in a limited set of activities and generally do not take insured deposits or engage in commercial lending. Importantly, national trust banks benefit from federal preemption of certain state laws that may apply to a state-chartered trust company, such as money transmitter licensing laws. The proposed activities of the five institutions include digital asset custody, settlement, clearing, transfer, escrow, staking, trade execution, and brokerage services; fiduciary, exchange, and payment agent services; stablecoin issuance; and the provision of services, including reserve asset custody, to affiliated stablecoin issuers. The Tier 1 capital requirements for these institutions range from $6.05 million to $25 million. Several other national trust bank charter applications remain pending, and a range of institutions is considering or actively preparing similar applications. The OCC has indicated its intent to process applications on their merits in a timely manner, generally within 120 days from the receipt of a complete application. This clearly signals an open window for credible, well-advised applicants who are interested in chartering or acquiring a national bank.
OCC Crypto-Focused Interpretive Letters
Building on earlier interpretive letters issued in 2020 and 2021, in 2025 the OCC issued several interpretive letters broadening the scope of digital asset activities expressly acknowledged as permissible for national banks.1
Interpretive Letter 1184: Cryptoasset Custody Services
In May 2025, the OCC confirmed that national banks and federal savings associations may engage in the purchase or sale of digital assets held in custody at the custodial customer’s direction. The OCC further confirmed that national banks and federal savings associations may outsource permissible digital asset activities, including custody and execution services, to third parties so long as appropriate third-party risk management practices are in place.
Interpretive Letter 1186: Paying Cryptoasset Network Fees and Holding Cryptoassets as Principal
In November 2025, the OCC confirmed that national banks may pay blockchain network fees (commonly known as “gas fees”) in connection with otherwise permissible activities and may hold digital assets as principal (i.e., on balance sheet) in amounts necessary to make such reasonably foreseeable payments. The OCC additionally clarified that national banks may hold digital assets as principal in amounts needed to test otherwise permissible digital asset platforms, whether internally developed or obtained from a third party.
Interpretive Letter 1188: Riskless Principal Transactions in Cryptoassets
In December 2025, the OCC confirmed that national banks may engage in riskless principal digital asset transactions on behalf of their customers. In such transactions, as distinct from agency transactions, a bank enters into two offsetting trades as principal, with the net result being that the bank generally does not hold digital assets in inventory and does not retain market exposure to the assets.
OCC Release of Nonobjection Requests
In March 2025, the OCC rescinded Interpretive Letter 1179, which required formal supervisory nonobjection (SNO) from the regulator before a national bank or federal savings association could undertake certain digital asset activities. Later, in connection with a report to Congress on debanking in December 2025,2 the OCC published a chart summarizing each formal SNO request submitted by an OCC-supervised institution under Interpretive Letter 1179 from the letter’s issuance on November 18, 2021, to its rescission on March 7, 2025, including the OCC’s response where applicable. Of the 21 SNO requests during this period, nine resulted in the OCC providing SNO. Eight of the nine granted requests involved the use of an internal blockchain to support financial transactions, and the remaining granted request involved the provision of traditional issuing and paying agent services to issuer clients, where those clients used a third-party clearinghouse’s private permissioned distributed ledger technology network to issue notes. Most of the remaining requests were withdrawn by the applicants in the face of agency resistance to expanded digital asset activities.
OCC Proposal to Clarify Chartering Authority for National Trust Banks
On January 8, the OCC issued a proposed rulemaking to clarify its longstanding authority to charter national banks limited to the operations of trust companies and activities related thereto, including to engage in nonfiduciary activities in addition to their fiduciary activities. The proposal would neither expand nor contract the OCC’s authority to charter a national bank but seeks to address ambiguity regarding how the OCC interprets its authority to charter banks under the National Bank Act. Comments on all aspects of the proposed rule are due 30 days after it is published in the Federal Register.
Federal Reserve Replacement of 2023 Section 9(13) Policy Statement
In February 2023, the Federal Reserve adopted a policy statement interpreting Section 9(13) of the Federal Reserve Act with respect to the Federal Reserve’s regulation of “novel and unprecedented activities” engaged in by state member banks. Section 9(13) of the Federal Reserve Act describes the manner in which activities of state member banks may be regulated and provides that the Federal Reserve may limit the activities of such banks to those permissible for national banks. The 2023 policy statement established a presumption that state member banks, whether insured or uninsured, would be limited to activities permissible for national banks or otherwise authorized by federal statute or FDIC regulation, even where broader activities were authorized under the laws of the state that chartered the bank. In practice, this limited the ability of state member banks to pursue novel or innovative activities, including certain digital asset activities.
In December 2025, the Federal Reserve rescinded the 2023 policy statement and withdrew its accompanying discussion of digital asset activities in response to an “evolving understanding of the risks of the crypto-asset sector” and a desire to facilitate innovation in the industry consistent with preserving the stability of the U.S. financial system. The Federal Reserve simultaneously adopted a new policy statement articulating a principles-based approach to the exercise of its authority under Section 9(13). Under the new framework, the Federal Reserve generally permits insured state member banks to engage in activities that are permitted for national banks or otherwise authorized by the FDIC for state banks (whether by regulation or special application). With respect to uninsured member banks seeking to engage in activities not permitted for national banks or otherwise authorized by the FDIC, the Federal Reserve emphasizes a supervisory assessment focused on risk, safety and soundness, and financial stability rather than categorical presumptions based on national bank powers. The new policy reflects a shift toward evaluating activities based on their risk profile and the institution’s ability to manage those risks, creating greater regulatory flexibility for member banks as they consider emerging business models, including those involving digital assets and payment innovation.
Federal Reserve Request for Information on New “Payment Accounts”
Federal Reserve master accounts provide institutions with the ability to hold balances at a Federal Reserve Bank and directly access services such as Fedwire and ACH. While traditional banks have long had such access, proposals for access by nontraditional or narrowly focused institutions have prompted heightened scrutiny from the Federal Reserve. This scrutiny reflects regulatory questions about eligibility, risk management, and the appropriate regulatory perimeter of the Federal Reserve System.
Against this backdrop, the Federal Reserve Board voted in December 2025 to seek public comment on a proposed special purpose “payment account” framework. These accounts, often referred to as “skinny master accounts,” would be more limited than traditional master accounts and would have the primary purpose of supporting the clearing and settling of payment activity. The payment account concept signals the Federal Reserve’s willingness to reconsider how access to core payment infrastructure might be structured for institutions pursuing innovative payment models, including those related to digital assets and tokenized payments, while limiting broader balance sheet, credit, or risk exposures traditionally associated with full master accounts. At the same time, the request for comment underscores that several questions remain unresolved, including eligibility standards, permissible uses, operational constraints, and the relationship between payment accounts and existing master account policies. How the Federal Reserve ultimately resolves these issues (e.g., by excluding ACH from the proposal) may have significant implications for banks, bank-adjacent entities, and payments-focused institutions seeking more direct participation in the U.S. payment system.
Treasury Seeks Input on GENIUS Act Implementation
In September 2025, the Treasury published an ANPRM seeking public comment on questions relating to the implementation of the GENIUS Act. The ANPRM invited comments across a broad set of issues that may inform Treasury’s forthcoming rulemaking under the GENIUS Act, including regulatory clarity, prohibitions on certain issuances and marketing, anti-money-laundering and sanctions obligations, the balance of state and federal oversight, comparability of foreign regulatory regimes, and tax implications.
Treasury’s ANPRM posed questions organized into key topic areas such as stablecoin issuers and service providers, illicit finance, foreign payment stablecoin issuers, taxation, insurance, and economic data and also sought input on definitions and potential safe harbors under the GENIUS Act. Commenters were encouraged to address how best to implement statutory provisions, including protections for consumers and financial stability, as well as Treasury’s role as chair of the Stablecoin Certification Review Committee. The comment period was extended to and closed on November 4, 2025.
As an ANPRM, Treasury’s notice does not create binding requirements but was intended to inform the development of future notices of proposed rulemaking and regulations under the GENIUS Act. Treasury’s solicitation of comments will be followed by one or more NPRMs addressing specific aspects of GENIUS Act implementation and shaping the stablecoin regulatory framework.
FDIC Issues NPRM on Bank-Issued Payment Stablecoins
In December 2025, the FDIC approved an NPRM that would establish application procedures for FDIC-supervised institutions (including state nonmember banks and state-chartered savings associations) seeking to issue payment stablecoins through an approved subsidiary under the GENIUS Act. The FDIC’s proposed rule is the FDIC’s first formal step in implementing the GENIUS Act’s stablecoin framework for bank-linked stablecoin issuance.
The NPRM outlines eligibility criteria, application requirements, review timelines, and appeal rights. Applicants would be required to submit detailed information regarding the business plan for stablecoin issuance, financial information, governance and control arrangements, and relevant information for the FDIC to evaluate risk controls and compliance readiness for stablecoin issuance.
Key open questions include how the FDIC will apply and evaluate safety and soundness standards in practice and how future rulemakings under the GENIUS Act will shape the broader regulatory environment for stablecoin issuance. For additional detail, see our previous Sidley Update.
1 See OCC Interpretive Letter 1186 (Nov. 18, 2025); OCC Interpretive Letter 1188 (Dec. 9, 2025).
2 See https://occ.gov/news-issuances/news-releases/2025/nr-occ-2025-114.html.
The environment has never been more favorable for existing banking organizations launching a digital asset business and those Fintech and other nonbank companies considering acquiring or chartering a full-service or limited-purpose bank in order to operate a digital asset business. At the end of 2025, U.S. banking regulators continued to take major steps signaling a considerably more open supervisory posture regarding digital asset activities.
First, in December, the Office of the Comptroller of the Currency (OCC) conditionally approved five applications to either newly charter or convert existing institutions into national trust banks that will engage in digital asset activities. Sidley represented FMR LLC, the parent company of Fidelity Investments, in obtaining an approval permitting the conversion of its New York limited purpose trust company into a national trust bank. These approvals built upon a series of interpretive letters released by the OCC in 2025 expanding the range of permissible digital asset activities for national banks as well as the OCC’s rescission of an earlier interpretive letter that required formal nonobjection from the regulator before a national bank or federal savings association could engage in certain digital asset activities. Finally, on January 8, the OCC issued a proposed rulemaking that would clarify, but not change the scope, of its authority to charter national trust banks.
Second, the Board of Governors of the Federal Reserve System (the Federal Reserve) (i) rescinded and replaced a 2023 policy statement regarding state member bank novel activities with a new, more permissive policy statement and (ii) published a request for information seeking public comment on a proposed special purpose Federal Reserve Bank “payment account” prototype (informally, a “skinny master account”) aimed at institutions focused on payments innovation.
Finally, the U.S. Department of the Treasury (Treasury) published an advance notice of proposed rulemaking (ANPRM) seeking public comment relating to the implementation of the Guiding and Establishing National Innovation for U.S. Stablecoins Act (GENIUS Act), while the Federal Deposit Insurance Corporation (FDIC) issued a notice of proposed rulemaking (NPRM) establishing an application process for subsidiaries of FDIC-supervised insured depository institutions to issue payment stablecoins under the GENIUS Act.
OCC Conditional Approvals for National Trust Bank Charters
On December 12, 2025, the OCC announced the conditional approval of five national trust bank charter applications from institutions proposing to offer digital asset products and services. Of the five applications, two were for de novo national trust bank charters, while three were for conversions from a state trust company to a national trust bank. National trust banks engage in a limited set of activities and generally do not take insured deposits or engage in commercial lending. Importantly, national trust banks benefit from federal preemption of certain state laws that may apply to a state-chartered trust company, such as money transmitter licensing laws. The proposed activities of the five institutions include digital asset custody, settlement, clearing, transfer, escrow, staking, trade execution, and brokerage services; fiduciary, exchange, and payment agent services; stablecoin issuance; and the provision of services, including reserve asset custody, to affiliated stablecoin issuers. The Tier 1 capital requirements for these institutions range from $6.05 million to $25 million. Several other national trust bank charter applications remain pending, and a range of institutions is considering or actively preparing similar applications. The OCC has indicated its intent to process applications on their merits in a timely manner, generally within 120 days from the receipt of a complete application. This clearly signals an open window for credible, well-advised applicants who are interested in chartering or acquiring a national bank.
OCC Crypto-Focused Interpretive Letters
Building on earlier interpretive letters issued in 2020 and 2021, in 2025 the OCC issued several interpretive letters broadening the scope of digital asset activities expressly acknowledged as permissible for national banks.1
Interpretive Letter 1184: Cryptoasset Custody Services
In May 2025, the OCC confirmed that national banks and federal savings associations may engage in the purchase or sale of digital assets held in custody at the custodial customer’s direction. The OCC further confirmed that national banks and federal savings associations may outsource permissible digital asset activities, including custody and execution services, to third parties so long as appropriate third-party risk management practices are in place.
Interpretive Letter 1186: Paying Cryptoasset Network Fees and Holding Cryptoassets as Principal
In November 2025, the OCC confirmed that national banks may pay blockchain network fees (commonly known as “gas fees”) in connection with otherwise permissible activities and may hold digital assets as principal (i.e., on balance sheet) in amounts necessary to make such reasonably foreseeable payments. The OCC additionally clarified that national banks may hold digital assets as principal in amounts needed to test otherwise permissible digital asset platforms, whether internally developed or obtained from a third party.
Interpretive Letter 1188: Riskless Principal Transactions in Cryptoassets
In December 2025, the OCC confirmed that national banks may engage in riskless principal digital asset transactions on behalf of their customers. In such transactions, as distinct from agency transactions, a bank enters into two offsetting trades as principal, with the net result being that the bank generally does not hold digital assets in inventory and does not retain market exposure to the assets.
OCC Release of Nonobjection Requests
In March 2025, the OCC rescinded Interpretive Letter 1179, which required formal supervisory nonobjection (SNO) from the regulator before a national bank or federal savings association could undertake certain digital asset activities. Later, in connection with a report to Congress on debanking in December 2025,2 the OCC published a chart summarizing each formal SNO request submitted by an OCC-supervised institution under Interpretive Letter 1179 from the letter’s issuance on November 18, 2021, to its rescission on March 7, 2025, including the OCC’s response where applicable. Of the 21 SNO requests during this period, nine resulted in the OCC providing SNO. Eight of the nine granted requests involved the use of an internal blockchain to support financial transactions, and the remaining granted request involved the provision of traditional issuing and paying agent services to issuer clients, where those clients used a third-party clearinghouse’s private permissioned distributed ledger technology network to issue notes. Most of the remaining requests were withdrawn by the applicants in the face of agency resistance to expanded digital asset activities.
OCC Proposal to Clarify Chartering Authority for National Trust Banks
On January 8, the OCC issued a proposed rulemaking to clarify its longstanding authority to charter national banks limited to the operations of trust companies and activities related thereto, including to engage in nonfiduciary activities in addition to their fiduciary activities. The proposal would neither expand nor contract the OCC’s authority to charter a national bank but seeks to address ambiguity regarding how the OCC interprets its authority to charter banks under the National Bank Act. Comments on all aspects of the proposed rule are due 30 days after it is published in the Federal Register.
Federal Reserve Replacement of 2023 Section 9(13) Policy Statement
In February 2023, the Federal Reserve adopted a policy statement interpreting Section 9(13) of the Federal Reserve Act with respect to the Federal Reserve’s regulation of “novel and unprecedented activities” engaged in by state member banks. Section 9(13) of the Federal Reserve Act describes the manner in which activities of state member banks may be regulated and provides that the Federal Reserve may limit the activities of such banks to those permissible for national banks. The 2023 policy statement established a presumption that state member banks, whether insured or uninsured, would be limited to activities permissible for national banks or otherwise authorized by federal statute or FDIC regulation, even where broader activities were authorized under the laws of the state that chartered the bank. In practice, this limited the ability of state member banks to pursue novel or innovative activities, including certain digital asset activities.
In December 2025, the Federal Reserve rescinded the 2023 policy statement and withdrew its accompanying discussion of digital asset activities in response to an “evolving understanding of the risks of the crypto-asset sector” and a desire to facilitate innovation in the industry consistent with preserving the stability of the U.S. financial system. The Federal Reserve simultaneously adopted a new policy statement articulating a principles-based approach to the exercise of its authority under Section 9(13). Under the new framework, the Federal Reserve generally permits insured state member banks to engage in activities that are permitted for national banks or otherwise authorized by the FDIC for state banks (whether by regulation or special application). With respect to uninsured member banks seeking to engage in activities not permitted for national banks or otherwise authorized by the FDIC, the Federal Reserve emphasizes a supervisory assessment focused on risk, safety and soundness, and financial stability rather than categorical presumptions based on national bank powers. The new policy reflects a shift toward evaluating activities based on their risk profile and the institution’s ability to manage those risks, creating greater regulatory flexibility for member banks as they consider emerging business models, including those involving digital assets and payment innovation.
Federal Reserve Request for Information on New “Payment Accounts”
Federal Reserve master accounts provide institutions with the ability to hold balances at a Federal Reserve Bank and directly access services such as Fedwire and ACH. While traditional banks have long had such access, proposals for access by nontraditional or narrowly focused institutions have prompted heightened scrutiny from the Federal Reserve. This scrutiny reflects regulatory questions about eligibility, risk management, and the appropriate regulatory perimeter of the Federal Reserve System.
Against this backdrop, the Federal Reserve Board voted in December 2025 to seek public comment on a proposed special purpose “payment account” framework. These accounts, often referred to as “skinny master accounts,” would be more limited than traditional master accounts and would have the primary purpose of supporting the clearing and settling of payment activity. The payment account concept signals the Federal Reserve’s willingness to reconsider how access to core payment infrastructure might be structured for institutions pursuing innovative payment models, including those related to digital assets and tokenized payments, while limiting broader balance sheet, credit, or risk exposures traditionally associated with full master accounts. At the same time, the request for comment underscores that several questions remain unresolved, including eligibility standards, permissible uses, operational constraints, and the relationship between payment accounts and existing master account policies. How the Federal Reserve ultimately resolves these issues (e.g., by excluding ACH from the proposal) may have significant implications for banks, bank-adjacent entities, and payments-focused institutions seeking more direct participation in the U.S. payment system.
Treasury Seeks Input on GENIUS Act Implementation
In September 2025, the Treasury published an ANPRM seeking public comment on questions relating to the implementation of the GENIUS Act. The ANPRM invited comments across a broad set of issues that may inform Treasury’s forthcoming rulemaking under the GENIUS Act, including regulatory clarity, prohibitions on certain issuances and marketing, anti-money-laundering and sanctions obligations, the balance of state and federal oversight, comparability of foreign regulatory regimes, and tax implications.
Treasury’s ANPRM posed questions organized into key topic areas such as stablecoin issuers and service providers, illicit finance, foreign payment stablecoin issuers, taxation, insurance, and economic data and also sought input on definitions and potential safe harbors under the GENIUS Act. Commenters were encouraged to address how best to implement statutory provisions, including protections for consumers and financial stability, as well as Treasury’s role as chair of the Stablecoin Certification Review Committee. The comment period was extended to and closed on November 4, 2025.
As an ANPRM, Treasury’s notice does not create binding requirements but was intended to inform the development of future notices of proposed rulemaking and regulations under the GENIUS Act. Treasury’s solicitation of comments will be followed by one or more NPRMs addressing specific aspects of GENIUS Act implementation and shaping the stablecoin regulatory framework.
FDIC Issues NPRM on Bank-Issued Payment Stablecoins
In December 2025, the FDIC approved an NPRM that would establish application procedures for FDIC-supervised institutions (including state nonmember banks and state-chartered savings associations) seeking to issue payment stablecoins through an approved subsidiary under the GENIUS Act. The FDIC’s proposed rule is the FDIC’s first formal step in implementing the GENIUS Act’s stablecoin framework for bank-linked stablecoin issuance.
The NPRM outlines eligibility criteria, application requirements, review timelines, and appeal rights. Applicants would be required to submit detailed information regarding the business plan for stablecoin issuance, financial information, governance and control arrangements, and relevant information for the FDIC to evaluate risk controls and compliance readiness for stablecoin issuance.
Key open questions include how the FDIC will apply and evaluate safety and soundness standards in practice and how future rulemakings under the GENIUS Act will shape the broader regulatory environment for stablecoin issuance. For additional detail, see our previous Sidley Update.
1 See OCC Interpretive Letter 1186 (Nov. 18, 2025); OCC Interpretive Letter 1188 (Dec. 9, 2025).
2 See https://occ.gov/news-issuances/news-releases/2025/nr-occ-2025-114.html.
In-brief analysis
Jan 9, 2026
In 2025, the wholesale U.S. natural gas spot price at the national benchmark Henry Hub in Louisiana averaged $3.52 per million British thermal units (MMBtu), based on data from LSEG Data. The 2025 average Henry Hub natural gas spot price increased 56% from the 2024 annual average, which—when adjusted for inflation—was the lowest on record. On a daily basis, the Henry Hub natural gas spot price ranged from $2.65/MMBtu to $9.86/MMBtu, reflecting a narrower range of daily prices compared with the previous year.
Read More ›
In-brief analysis
Jan 7, 2026

The U.S. retail price for regular grade gasoline averaged $3.10 per gallon (gal) in 2025, $0.21/gal less than in 2024. This year marks the third consecutive year of declining nominal retail gasoline prices, according to data from our Gasoline and Diesel Fuel Update.
Read More ›
In-brief analysis
Jan 5, 2026

Crude oil prices generally declined in 2025 with supplies in the global crude oil market exceeding demand. Crude oil inventory builds in China muted some of the price decline. Events such as Israel’s June 13 strikes on Iran and attacks between Russia and Ukraine targeting oil infrastructure periodically supported prices.
Read More ›
In-brief analysis
Dec 22, 2025

Source: U.S. Energy Information Administration
Below is a list featuring some of our most popular and favorite articles from 2025. We will resume regular Today in Energy publications on January 5, 2026. Thanks for your continued readership of Today in Energy.
Read More ›
In-brief analysis
Dec 19, 2025

Each month we publish estimates of key global oil market indicators that affect crude oil prices and movements in our Short-Term Energy Outlook (STEO). Among the most important indicators for global crude oil markets are estimates of OPEC’s effective crude oil production capacity and surplus production capacity, as well as any disruptions to liquid fuels production. Low surplus production capacity among OPEC countries can put upward pressure on crude oil prices in the event of unplanned supply disruptions or strong growth in global oil demand.
Read More ›
In-brief analysis
Dec 17, 2025

We forecast that global crude oil production will increase by 0.8 million barrels per day (b/d) in 2026, with supply from Brazil, Guyana, and Argentina accounting for 0.4 million b/d of the expected global growth forecast in our December Short-Term Energy Outlook (STEO). Global crude oil production growth since 2023 has been driven by countries outside of OPEC+.
Read More ›
In-brief analysis
Dec 15, 2025

Our estimates for residential energy expenditures this winter (November 2025 through March 2026) have increased since the publication of our initial Winter Fuels Outlook forecasts in mid-October. We now expect a colder winter, and our retail energy price forecasts have risen, especially for natural gas and propane.
Read More ›
In-brief analysis
Dec 12, 2025

Read More ›
In-brief analysis
Dec 10, 2025

Critical minerals, such as copper, cobalt, and silicon, are vital for energy technologies, but most critical minerals markets are less transparent than mature energy markets, such as crude oil or coal. Like other energy markets, many supply-side and demand-side factors influence pricing for these energy-relevant critical minerals, but critical minerals supply chains contain numerous data gaps.
Read More ›
In-brief analysis
Dec 8, 2025

Higher average daily wholesale electricity prices between January and November 2025 may be improving the operational competitiveness of some natural gas- and coal-fired generators in the PJM Interconnection compared with the same period in 2024. PJM is the largest wholesale electricity market in the United States. The spark and dark spreads, common metrics for estimating the profitability of natural gas- and coal-fired electric generators, have both increased over the past two years.
Read More ›
In-brief analysis
Dec 5, 2025

Read More ›
In-brief analysis
Dec 3, 2025

Data source: Bloomberg L.P.
Note: Data through November 26, 2025. All crack spreads are calculated against the Dated Brent crude oil spot price.
Global refinery margins for diesel have widened since late October and increased to their highest level all year, following refinery outages in Russia and in the Middle East and new sanctions on Russia’s crude oil, leading to limited refinery production and a decreased global diesel supply. The impact was most pronounced in the Atlantic Basin, contributing to higher prices at the Amsterdam, Rotterdam, Antwerp (ARA) shipping hub, a key benchmark for European prices, as well as at New York Harbor and the U.S. Gulf Coast. The higher global prices also affected prices in the United States because U.S. refiners can sell into both domestic and international markets.
Read More ›
In-brief analysis
Dec 1, 2025

U.S. electricity customers experienced an average of 11 hours of electricity interruptions in 2024, or nearly twice as many as the annual average experienced in the decade before, according to our Electric Power Annual 2024 report. Major events such as Hurricanes Beryl, Helene, and Milton accounted for 80% of the hours without electricity in 2024.
Read More ›
In-brief analysis
Nov 26, 2025

On the Monday before Thanksgiving, the U.S. retail price for regular-grade gasoline averaged $3.06 per gallon (gal), just 2 cents/gal higher than the same time last year. After adjusting for inflation, however, this year marks the lowest average gasoline price for the Monday before the Thanksgiving holiday weekend since 2020, when the pandemic disrupted gasoline demand and travel plans.
Read More ›
In-brief analysis
Nov 24, 2025

Although natural gas generation still provides more electricity than any other source in California, electricity generation from natural gas has decreased over the past several years while generation from solar has increased.
Read More ›

New capability delivers actionable insights from card-present transactions
MILWAUKEE–(BUSINESS WIRE)–
Fiserv, Inc. (NASDAQ: FISV), a leading global provider of payments and financial services technology, today announced the launch of Unknown Shopper from Fiserv, a new analytics capability designed to help merchants and their marketing partners better understand in-store customer behavior and build actionable customer segments from card-present transactions.
Unknown Shopper enables merchants to unlock value from in-store payment activity by transforming payment data into actionable insights that support more relevant engagement, and better customer experiences. Built on Fiserv’s payments intelligence platform and informed by billions of historical transactions, the solution helps merchants across retail, restaurant, and fuel environments—industries where insight into card-present transactions has historically been limited—gain deeper visibility into purchasing patterns.
Addressing an in-store payments challenge
In traditional card-present environments, merchants often have limited visibility of their customers unless a transaction is tied to a loyalty program. As a result, a significant portion of in-store activity remains difficult to analyze, measure, or activate. Unknown Shopper helps close this gap by enriching transaction data with demographic information, enabling merchants to better understand in-store purchasing patterns.
Unknown Shopper allows businesses to link transactions to actionable customer segments based on real purchase behavior, even without a loyalty program. To that end, the solution helps businesses, with the assistance of their marketing partners, to:
“Unknown Shopper allows merchants to translate in-store transaction data into meaningful insights and customer segments—helping them serve their customers more effectively,” said Prasanna Dhore, Chief Data Officer, Fiserv.
Unknown Shopper is now available to merchants and marketing agencies. Learn more about Unknown Shopper.
Fiserv will demonstrate the solution at NRF 2026: Retail’s Big Show, January 11-13, at the Jacob J. Javits Convention Center (Booth #5451).
About Fiserv
Fiserv, Inc. (NASDAQ: FISV), a Fortune 500 company, moves more than money. As a global leader in payments and financial technology, the company helps clients achieve best-in-class results through a commitment to innovation and excellence in areas including account processing and digital banking solutions; card issuer processing and network services; payments; e-commerce; merchant acquiring and processing; and Clover®, the world’s smartest point-of-sale system and business management platform. Fiserv is a member of the S&P 500® Index, one of TIME Magazine’s Most Influential Companies™ and one of Fortune® World’s Most Admired Companies™. Visit fiserv.com and follow on social media for more information and the latest company news.
FISV-G
View source version on businesswire.com: https://www.businesswire.com/news/home/20260109022168/en/
Media Relations:
Chase Wallace
Director, Communications
Fiserv, Inc.
+1 470-481-2555
chase.wallace@fiserv.com
Additional Contact:
Melissa Moritz
Vice President, External Communications
Fiserv, Inc.
+1 516-410-1188
melissa.moritz@fiserv.com
Source: Fiserv, Inc.
Released January 9, 2026
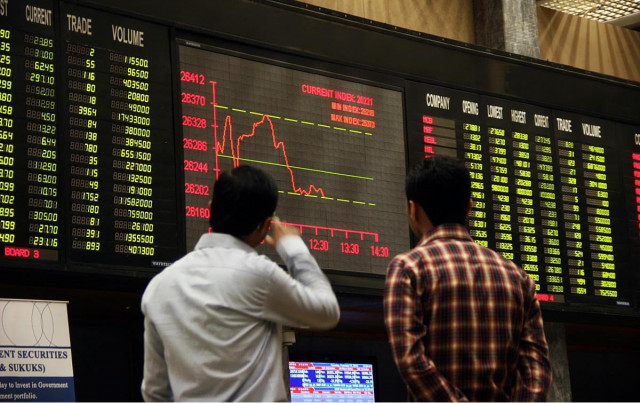
The Pakistan Stock Exchange (PSX) ended the week on a bearish note as investors opted to book profits ahead of the weekend, dragging the benchmark KSE-100 Index into negative territory for most of the session.
“KSE-100 Index largely traded in the negative zone during the trading session to close at 184,410 level (down by -0.61%), as investors preferred to book profits before the weekend,” Topline market review noted.
Top negative contributions to the index came from HUBC, LUCK, ENGROH, NBP, EFERT and OGDC, as they cumulatively contributed -596 points to the index.
Traded value-wise, FFC (PKR.2.14bn), PPL (PKR.1.88bn), PAEL (PKR.1.83bn), FFL (PKR.1.68bn), NBP (PKR.1.67bn) and SYS (PKR.1.64bn) dominated the trading activity.
Traded volume and value for the day stood at 1.02bn shares and PKR.52.76bn, respectively.
BEIJING, Jan. 9 — Chinese Premier Li Qiang on Friday chaired a State Council executive meeting during which arrangements were made to implement a package of policies that will boost domestic demand by leveraging coordinated fiscal and financial measures.
The meeting noted that the package is an important measure to expand effective demand and innovate the approaches of macro-control.
Efforts should be made to refine loan interest subsidy policies for businesses in the services sector and personal consumption, aiming to expand the supply of high-quality services and boost household purchasing power, the meeting said.
It said that loan interest subsidy policies will be implemented for micro, small and medium-sized enterprises, with an eye to bolstering private investment, and to lowering financing thresholds and costs for companies.
It stressed that basic public services should be provided based on an individual’s place of permanent residence. Efforts will be made to address the most pressing concerns of permanent residents without a local household registration, and on improving their access to education, healthcare and employment services.
The meeting also reviewed and approved a draft revision to China’s regulations for nature reserves.

Released: 2026-01-09
In December, employment was little changed (+8,200; 0.0%) and the employment rate held steady at 60.9%.
The unemployment rate rose 0.3 percentage points to 6.8%, as more people searched for work.
Employment rose among people aged 55 and older (+33,000; +0.8%), while it fell among youth aged 15 to 24 (-27,000; -1.0%).
There were more people working in health care and social assistance (+21,000; +0.7%) as well as in ‘other services’ such as personal and repair services (+15,000; +2.0%). At the same time, fewer people were employed in professional, scientific and technical services (-18,000; -0.9%), accommodation and food services (-12,000; -1.0%), and utilities (-5,300; -3.0%).
Employment was up in Quebec (+16,000; +0.3%) while it fell in Alberta (-14,000; -0.5%) and Saskatchewan (-4,000; -0.6%). There was little employment change in the other provinces.
Average hourly wages among employees increased 3.4% (+$1.23 to $37.06) on a year-over-year basis in December, following growth of 3.6% in November (not seasonally adjusted).




Employment was little changed (+8,200; 0.0%) in December. This followed three consecutive monthly increases in September, October and November (totalling 181,000; +0.9%). The employment rate—the percentage of the population aged 15 years and older who are employed—held steady at 60.9% in December.
Full-time employment rose by 50,000 (+0.3%) in December while part-time employment fell by 42,000 (-1.1%). The decline in part-time work in the month partially offsets a cumulative gain of 148,000 (+3.9%) in October and November. Over the 12 months to December 2025, part-time employment rose at a faster pace (+2.6%; +99,000) than full-time employment (+0.7%; +128,000).
In December, there was little change in the number of private and public sector employees, as well as in the number of self-employed workers.
The unemployment rate rose 0.3 percentage points to 6.8% in December, as more people searched for work. The increase in the unemployment rate in December partially offsets a cumulative decline of 0.6 percentage points in the previous two months.


There were 1.6 million people unemployed in December, an increase of 73,000 (+4.9%) in the month.
The participation rate—the proportion of the population aged 15 and older who were employed or looking for work—rose by 0.3 percentage points to 65.4%. On a year-over-year basis, the labour force participation rate was unchanged in December.
The unemployment rate for youth aged 15 to 24 rose 0.5 percentage points to 13.3% in December, as fewer youth were employed (-27,000; -1.0%). Labour market conditions had previously improved for youth in October and November, with employment rising (+70,000; +2.6%) and the youth unemployment rate falling 1.9 percentage points over this period.
In 2025, the labour market faced headwinds, in part due to the economic uncertainty introduced by the threat or imposition of tariffs on exports to the United States. The impacts of tariff-related trade uncertainty in 2025 have been detailed in recent Statistics Canada studies (see: United States tariffs and Canadian labour market trends and Recent employment trends in industries dependent on U.S. demand).
From January to August, there was virtually no net employment growth in Canada. The employment rate trended down over this period, from 61.1% in January to a low of 60.5% in August. At the same time, the unemployment rate rose and reached a high of 7.1% in August—the highest level since May 2016 (excluding 2020 and 2021 during the COVID-19 pandemic).
The increase in the unemployment rate over the first eight months of 2025 mostly reflected slower hirings. The job finding rate—that is, the proportion of job seekers who found work from one month to the next—was 18.1% on average between January and August 2025. This was lower compared with the corresponding periods in 2024 (21.0%) and between 2017 and 2019 (24.0%), prior to the pandemic (not seasonally adjusted).
At the same time, layoff rates were similar to historical levels during this period. On average between January and August, the proportion of employed people who had become unemployed due to a layoff from one month to the next was 0.8%. This was identical to the average observed for the corresponding months in 2024 and between 2017 and 2019 (not seasonally adjusted).
Job vacancies also fell through most of 2025. Based on the Job Vacancy and Wage Survey, job vacancies were down by 56,000 (-10.2%) on a year-over-year basis in the third quarter of 2025. Just over one-quarter (27.1%) of job vacancies were long-term (meaning that recruitment efforts had been ongoing for 90 days or more), down from 31.6% during the third quarter of 2024 (not seasonally adjusted). This indicates that in addition to having fewer job vacancies, employers had fewer difficulties filling available positions compared with previous quarters.
More difficult labour market conditions in 2025 were most evident among youth. The youth unemployment rate reached a high of 14.7% in September, the highest rate since September 2010 (excluding 2020 and 2021). Students also faced a difficult summer job market in 2025. The unemployment rate for returning students was 17.9% on average between May and August—the highest rate since the summer of 2009 (when it was 18.0%), excluding 2020.
Labour market conditions improved in the final months of the year. Employment rose by 181,000 (+0.9%) from August to November, before holding steady in December. The employment rate increased over this period and stood at 60.9% at the end of the year, little changed from December 2024. The unemployment rate also fell to 6.5% in November 2025, its lowest level for the year, before increasing to 6.8% in December, as more people searched for work.


In December, among core-aged people (25 to 54 years old), increases in the number of people searching for work led to a rise in the unemployment rate for men (+0.4 percentage points to 6.0%) and women (+0.4 percentage points to 5.9%). On a year-over-year basis, the unemployment rate was up 0.4 percentage points for core-aged men, while it was little changed for core-aged women.
For people aged 55 and older, the unemployment rate fell 0.2 percentage points to 5.1% in December as employment rose by 33,000 (+0.8%). On a year-over-year basis, the unemployment rate for people aged 55 and older was unchanged.
Employment in health care and social assistance increased by 21,000 (+0.7%) in December, building on growth of 46,000 (+1.6%) recorded in November. This brought the net increase in health care and social assistance over the 12 months ending in December to 85,000 (+3.0%). On a year-over-year basis, employment in health care and social assistance grew faster among private sector employees (+6.9%) than among self-employed workers (+3.0%) (not seasonally adjusted). There was essentially no change in employment among public sector workers in health care and social assistance over the period.


On the other hand, there were fewer people employed in professional, scientific and technical services in December (-18,000; -0.9%), the first decrease since August. Despite the monthly decline, employment in the industry was little changed in December compared with 12 months earlier.
In December, employment increased by 16,000 (+0.3%) in Quebec, the first significant gain in the province since June 2025. The unemployment rate increased 0.3 percentage points to 5.4% in December as more people searched for work. Over the 12 months to December 2025, employment in the province was up by 45,000 (+1.0%), a slower rate of growth than in the 12 months ending in December 2024 (+1.6%; +72,000).
Employment declined in Alberta in December (-14,000; -0.5%), partly offsetting an increase of 29,000 in November. On a year-over-year basis, employment in Alberta was up by 58,000 (+2.3%) in December. The unemployment rate in Alberta was 6.8%, on par with the rate recorded at the end of 2024 (6.7%).


Employment also fell in Saskatchewan in December (-4,000; -0.6%), and the unemployment rate in the province increased 0.9 percentage points to 6.5%.
In Ontario, employment was little changed for the second consecutive month in December. Over the 12 months ending in December 2025, employment rose at a slower pace (+0.9%; +72,000), compared with the 12 months ending in 2024 (+2.1%; +165,000). With more Ontarians searching for work, the unemployment rate in the province increased 0.6 percentage points to 7.9% in December 2025, 0.4 percentage points higher than at year-end in 2024 (7.5%).
Digital platform employment is a form of work that can be flexible and easy to access, though it typically offers short-term tasks and limited job security. As one of the core components of the gig economy, this type of work involves paid work organized through websites or apps that connect workers with clients and often oversee or organize the work process.
In December 2025, 667,000 Canadians (2.3% of the population aged 15 to 69) had done paid work through a digital platform in the previous 12 months, little changed compared with December 2024 (671,000; 2.3%). These workers provided services; rented out accommodation, goods or equipment; or sold goods through websites or apps that coordinated their work activities or managed payments.
The most common types of digital platform employment that Canadians did in the 12 months to December remained the delivery of food or other goods (272,000 people), personal transport services (184,000 people) and selling goods online with the specific purpose of earning income (92,000 people).
Digital platform employment is often taken up as a secondary activity or done sporadically. Among people who had worked through an internet platform or app in the 12 months to December 2025, less than one-quarter (21.8%) were doing so as part of their main job or business in December.
Further, less than half (45.6%) of digital platform workers reported that the main reason they had started working through an internet platform or app was to supplement income from a main job or to earn extra money. Other common main reasons included difficulty finding other work (15.4%) or flexible working hours or convenience (14.2%).
Among recent immigrants (those who had landed in Canada in the previous five years), 8.4% had worked through an internet platform or app in the 12 months to December 2025. This was about 6 times higher than the corresponding proportion among people born in Canada (1.5%). Compared with a year earlier, the proportion was up 2.7 percentage points for recent immigrants but little changed (-0.1 percentage points) for people born in Canada.
On January 1, 2016, the world officially began implementation of the 2030 Agenda for Sustainable Development—the United Nations’ transformative plan of action that addresses urgent global challenges over the next 15 years. The plan is based on 17 specific sustainable development goals.
The Labour Force Survey is an example of how Statistics Canada supports the reporting on the Global Goals for Sustainable Development. This release will be used in helping to measure the following goals:



The Labour Force Survey (LFS) estimates for December reflect labour market conditions during the reference week of December 7 to 13, 2025.
The sample size of the LFS is approximately 65,000 households, representing over 100,000 respondents each month. For more information, see the Guide to the Labour Force Survey.
This analysis focuses on differences between estimates that are statistically significant at the 68% confidence level. Monthly estimates may show more sampling variability than trends observed over longer periods. For more information, see “Interpreting Monthly Changes in Employment from the Labour Force Survey.”
LFS estimates at the Canada level do not include the territories.
The LFS estimates are the first in a series of labour market indicators released by Statistics Canada, which includes indicators from programs such as the Survey of Employment, Payrolls and Hours (SEPH); Employment Insurance Statistics; and the Job Vacancy and Wage Survey. For more information on the conceptual differences between employment measures from the LFS and those from the SEPH, refer to section 8 of the Guide to the Labour Force Survey.
The employment rate is the number of employed people as a percentage of the population aged 15 years and older. The rate for a particular group (for example, youth aged 15 to 24 years) is the number employed in that group as a percentage of the population for that group.
The unemployment rate is the number of unemployed people as a percentage of the labour force (employed and unemployed).
The participation rate is the number of employed and unemployed people as a percentage of the population aged 15 years and older.
Full-time employment consists of persons who usually work 30 hours or more per week at their main or only job.
Part-time employment consists of persons who usually work less than 30 hours per week at their main or only job.
Total hours worked refers to the number of hours actually worked at the main job by the respondent during the reference week, including paid and unpaid hours. These hours reflect temporary decreases or increases in work hours (for example, hours lost due to illness, vacation, holidays or weather; or more hours worked due to overtime).
This release refers to the gender of a person. The category “men” includes men, as well as some non-binary persons. The category “women” includes women, as well as some non-binary persons. Given that the non-binary population is small, data aggregation to a two-category gender variable is necessary to protect the confidentiality of responses provided.
Unless otherwise stated, estimates presented in this release are seasonally adjusted, which facilitates comparisons by removing the effects of typical seasonal variations. For more information on seasonal adjustment, see Seasonally adjusted data – Frequently asked questions.
The LFS target population includes all persons aged 15 years and older whose usual place of residence is in Canada, with some exceptions (those living on reserves, full-time members of the regular Armed Forces and persons living in institutions). The target population includes temporary residents—that is, those with a valid work or study permit, their families, and refugee claimants—as well as permanent residents (landed immigrants) and the Canadian-born.
Information gathered from LFS respondents is weighted to represent the survey target population using population calibration totals. These totals are updated each month, using the most recently available information on population changes derived from Canada’s official population estimates, with minor adjustments being made to reflect the LFS target population.
While the LFS population totals are generally aligned with official demographic estimates, the official estimates should be considered the official measure of population change in Canada. More information on how population totals in the LFS are calculated can be found in the article “Interpreting population totals from the Labour Force Survey.”
Every 10 years, the LFS sample is redesigned to reflect changes in population characteristics and updated geographical boundaries. The updated sample design—based on the 2021 Census population characteristics and the 2021 Standard Geographical Classification—was phased in from April to September 2025. For more information, see Section 4 of the Guide to the Labour Force Survey.
Data for the Labour Market Indicators program are now available for December 2025.
On January 26, 2026, revised seasonally adjusted Labour Force Survey estimates for January 2023 to December 2025 will be released. This is a regular process done each year to incorporate the latest seasonal factors and results in minor changes to some recent estimates.
The next release of the LFS will be on February 6, 2026. January 2026 data will reflect labour market conditions during the week of January 11 to 17.
More information about the concepts and use of the Labour Force Survey is available online in the Guide to the Labour Force Survey (71-543-G).
The product “Labour Force Survey in brief: Interactive app” (14200001) is also available. This interactive visualization application provides seasonally adjusted estimates by province, gender, age group and industry.
The product “Labour Market Indicators, by province and census metropolitan area, seasonally adjusted” (71-607-X) is also available. This interactive dashboard provides customizable access to key labour market indicators.
The product “Labour Market Indicators, by province, territory and economic region, unadjusted for seasonality” (71-607-X) is also available. This dynamic web application provides access to labour market indicators for Canada, provinces, territories and economic regions.
The product “Labour market indicators, census metropolitan areas, census agglomerations and self-contained labour areas: Interactive dashboard” (71-607-X) is also available. This dashboard allows users to visually explore the estimates using an interactive map as well as time series charts and tables.
The product Labour Force Survey: Public Use Microdata File (71M0001X) is also available. This public use microdata file contains non-aggregated data for a wide variety of variables collected from the Labour Force Survey. The data have been modified to ensure that no individual or business is directly or indirectly identified. This product is for users who prefer to do their own analysis by focusing on specific subgroups in the population or by cross-classifying variables that are not in our catalogued products.
For more information, or to enquire about the concepts, methods or data quality of this release, contact us (toll-free 1-800-263-1136; 514-283-8300; infostats@statcan.gc.ca) or Media Relations (statcan.mediahotline-ligneinfomedias.statcan@statcan.gc.ca).

A look into the Cleethorpes of yesteryear has been unearthed during ongoing works to an historic building.
Nearly 250 years ago, the Dolphin Hotel, or the “Cleethorpes Hotel” as it was known at the time came into being, and it’s having a full make-over, partly thanks to The National Lottery Heritage Fund.
The Dolphin Hotel sits on the corner of Market Street and Alexandra Road in the seaside resort of Cleethorpes, and over the years has played a major role in the history and development of the town.
To support this increase in popularity, the Cleethorpes Hotel was built, with a major rebuild (and re-name) in the 1820s in response to growing visitor numbers to the town.
The building has been a hotel, restaurant, oyster bar, café and in more recent years, various nightclubs, and now the current owners are starting a major project to restore the building.
The restoration of the external features is being supported by the Townscape Heritage Project for Cleethorpes (TH) grant initiative. Grant funding is supported by North East Lincolnshire Council, the Heritage Fund and matched with third party funding from the freeholder.
The TH has already seen several buildings in the resort having the facades, shop fronts and heritage balconies restored. The Mermaid building on the North Promenade was also part of this programme.
The restoration work at the Dolphin includes:
During the restoration of the doors, a part of Cleethorpes history has been uncovered above the Main Entrance. Removal of the modern signage above the door revealed a marker labelled FP with the numbers 46 and 4, and this is thought to have been a point of information for the fire service in the 1900s.
FP is likely to represent Fire Point, or Fire Plug. These were an early form of fire hydrant and marks the distance in feet and inches from the sign to where the water supply was.
In modern times, we are familiar with fire hydrants where the Fire Service can connect to source water. However, in years gone by the Firemen were directed to an area where they would need to dig down to the water source, which was usually a mains water pipe. Once they’d finished using the pipe, they would often plug it with a piece of wood.
Therefore, what FP 46-4 is likely to represent is that the source of water closest to The Dolphin in an emergency was 46 feet and 4 inches away from the Main Entrance.
However, this is not the only FP marker that has been uncovered in Cleethorpes. On the nearby Empire, there is another example above the main entrance which show 14 feet and 1 inch.
Councillor Philip Jackson, Leader and Portfolio Holder for Economy, Regeneration, Devolution and Skills, said: “It’s great to see some of the original heritage come to life as we go through this programme of works.
“As part of the heritage led regeneration in Cleethorpes, it is important that we preserve the historic features that made Cleethorpes what it is today.”