Important DisclaimersFXEmpire is owned and operated by Empire Media Network LTD., Company Registration Number 514641786, registered at 7 Jabotinsky Road, Ramat Gan 5252007, Israel. The content provided on this website includes general news and publications, our personal analysis and opinions, and materials provided by third parties. This content is intended for educational and research purposes only. It does not constitute, and should not be interpreted as, a recommendation or advice to take any action, including making any investment or purchasing any product. Before making any financial decision, you should conduct your own due diligence, exercise your own discretion, and consult with competent advisors. The content on this website is not personally directed to you, and we do not take into account your individual financial situation or needs. The information contained on this website is not necessarily provided in real time, nor is it guaranteed to be accurate. Prices displayed may be provided by market makers and not by exchanges. Any trading or other financial decision you make is entirely your own responsibility, and you must not rely solely on any information provided through the website. FXEmpire does not provide any warranty regarding the accuracy, completeness, or reliability of any information contained on the website and shall bear no responsibility for any trading losses you may incur as a result of using such information. The website may include advertisements and other promotional content. FXEmpire may receive compensation from third parties in connection with such content. FXEmpire does not endorse, recommend, or assume responsibility for the use of any third-party services or websites. Empire Media Network LTD., its employees, officers, subsidiaries, and affiliates shall not be liable for any loss or damage resulting from your use of the website or reliance on the information provided herein.Risk DisclaimersThis website contains information about cryptocurrencies, contracts for difference (CFDs), and other financial instruments, as well as about brokers, exchanges, and other entities trading in such instruments. Both cryptocurrencies and CFDs are complex instruments and involve a high risk of losing money. You should carefully consider whether you understand how these instruments work and whether you can afford to take the high risk of losing your money. FX Empire encourages you to conduct your own research before making any investment decision and to avoid investing in any financial instrument unless you fully understand how it works and the risks involved.
Category: 3. Business
-

IMF Executive Board Completes the Fourth Review under the Extended Fund Facility and First Review under the Resilience and Sustainability Facility Arrangements for Jordan
Washington, DC: The Executive Board of the International Monetary Fund (IMF) today completed the fourth review of the arrangement under the Extended Fund Facility (EFF) and the first review of the Resilience and Sustainability Facility (RSF) arrangement. Jordan’s four-year EFF arrangement, with access amounting to SDR 926.37 million (about US$1.3 billion, equivalent to 270 percent of Jordan’s quota in the IMF), was approved by the IMF Executive Board on January 10, 2024 (see Press Release No. 24/004). This decision allows for an immediate purchase of an amount equivalent to SDR 97.784 million (about US$130 million), bringing the total purchases under the EFF arrangement to the equivalent of SDR 535.238 million (about US$733 million). In addition, the Resilience and Sustainability Facility (RSF) for Jordan was approved on June 25, 2025 (see Press Release No. 25/221), with access to SDR 514.65 million (about US$700 million, equivalent to 150 percent of Jordan’s quota). The Board’s decision will also allow the disbursement of SDR 79.182 million (about US$110 million) under the RSF.
Jordan’s economy remains resilient, supported by sound macroeconomic policies and strong international backing. Growth accelerated to 2.7 percent in the first half of 2025 and is expected to reach 3 percent in the coming years, aided by major investment projects, deeper regional integration, and sustained implementation of structural reforms. Inflation stays anchored at about 2 percent, and the current account deficit is projected to narrow to below 5 percent of GDP over the medium term. The banking sector is stable, and international reserves are strong.
Fiscal performance remains in line with program targets, with robust revenue collection and current spending discipline. The authorities are committed to reducing public debt to 80 percent of GDP by 2028 through gradual fiscal consolidation and further actions to lower the losses of public utilities, while protecting social and development spending.
The authorities are determined to step up the pace of structural reforms to achieve stronger growth and generate more jobs. Reforms are advancing to boost investment, foster competition, improve labor market flexibility, and strengthen the social safety net, alongside digitalization of government services.
Progress under the RSF continues, with measures addressing vulnerabilities in water and electricity sectors and enhancing health emergency preparedness. The two RSF Reform Measures scheduled for this review have been completed.
Following the Executive Board discussion, Kenji Okamura, Deputy Managing Director and Chair, made the following statement:
“Jordan’s continued macroeconomic stability and resilience amid persistent external headwinds are a testament to the authorities’ steadfast pursuit of sound policies, aided by strong international support. Growth continues to recover, inflation remains low, and reserve buffers are strong. In the context of lingering regional tensions and global uncertainty, the authorities continued commitment to sound fiscal and monetary policies to safeguard macroeconomic stability is important.
“The authorities continue to make progress on gradual and growth-friendly fiscal consolidation. The recalibrated fiscal stance for 2026 is appropriate. Gradual fiscal consolidation, supported by the authorities’ Medium-Term Revenue Strategy and enhanced spending efficiency would help to place public debt on a downward path, while protecting social and capital spending. Efforts to maintain the long-term financial sustainability of the pension system and improve the efficiency and financial viability of public utilities are crucial.
“Monetary policy remains appropriately focused on safeguarding monetary and financial stability and supporting the exchange rate peg that continues to serve Jordan well. Jordan’s banking sector remains healthy, and the central bank continues to strengthen its systemic risk analysis, financial sector oversight, and crisis management. Ongoing efforts to further strengthen the effectiveness of the AML/CFT framework are welcome.
“Accelerated structural reforms are crucial to create a dynamic and resilient private sector and foster job-rich growth. The authorities are focused on measures to improve the business environment, promote competition, enhance labor market flexibility to address youth unemployment and low female labor force participation, and attract private investment. Strong and timely donor support remains essential to help Jordan navigate the challenging external environment and meet its development objectives, while shouldering the cost of hosting a large number of refugees.
“The solid progress of implementing the reform measures under the Resilience and Sustainability Facility will help to support the authorities’ efforts to address long-term economic vulnerabilities and strengthen Jordan’s balance of payments stability.”
Jordan: Selected Economic Indicators, 2024-2027
2024
2025
2026
2027
Proj.
Proj.
Proj.
Output and Prices
(Annual percentage change, unless otherwise noted)
Real GDP growth
2.5
2.7
2.9
3.0
GDP deflator
1.9
2.3
2.4
2.3
Nominal GDP (JD billions)
41.6
43.7
46.1
48.6
Consumer price inflation (annual average)
1.6
1.9
2.2
2.2
Unemployment rate (percent) 1/
21.4
…
…
…
Fiscal Operations
(in percent of GDP, unless otherwise noted)
Revenue and grants
22.7
22.8
23.6
23.9
Of which: grants
1.7
1.7
1.6
1.4
Expenditure 2/
28.5
28.1
28.3
28.2
Overall central government balance 3/
-5.9
-5.3
-4.8
-4.3
Primary government balance (excluding grants)
-2.6
-1.9
-1.3
-0.5
Combined public sector balance 4/
-4.0
-3.2
-2.6
-1.6
Government and guaranteed gross debt 5/
106.1
108.6
108.1
108.0
Government and guaranteed gross debt, net of SSC’s holdings 5/
82.1
83.4
82.0
81.3
External Sector
(in percent of GDP, unless otherwise noted)
Current account balance (including grants)
-5.8
-5.1
-5.7
-5.5
Foreign Direct Investment
2.7
3.0
3.0
3.0
Gross usable international reserves ($ billions) 6/
20.3
20.7
21.4
21.8
In months of imports
7.7
7.3
7.2
7.0
In percent of the IMF’s Reserve Adequacy Metric
112
107
105
104
Sources: Jordanian authorities; and Fund staff estimates and projections.
1/ Unemployment rate for Jordanians only (excluding foreign residents).
2/ Includes other use of cash (i.e. off-budget expenditures).
3/ Includes statistical discrepancy.
4/ Defined as the sum of the primary central government balance (excl. grants and transfers to NEPCO and WAJ), NEPCO operating balance, and consolidated water sector balance.
5/ Government’s direct and guaranteed debt (including NEPCO and WAJ debt). SSC stands for Social Security Corporation.
6/ Including gold and excluding commercial banks’ FX deposits at the CBJ, bilateral accounts, and forward contracts. Including RSF.
Continue Reading
-
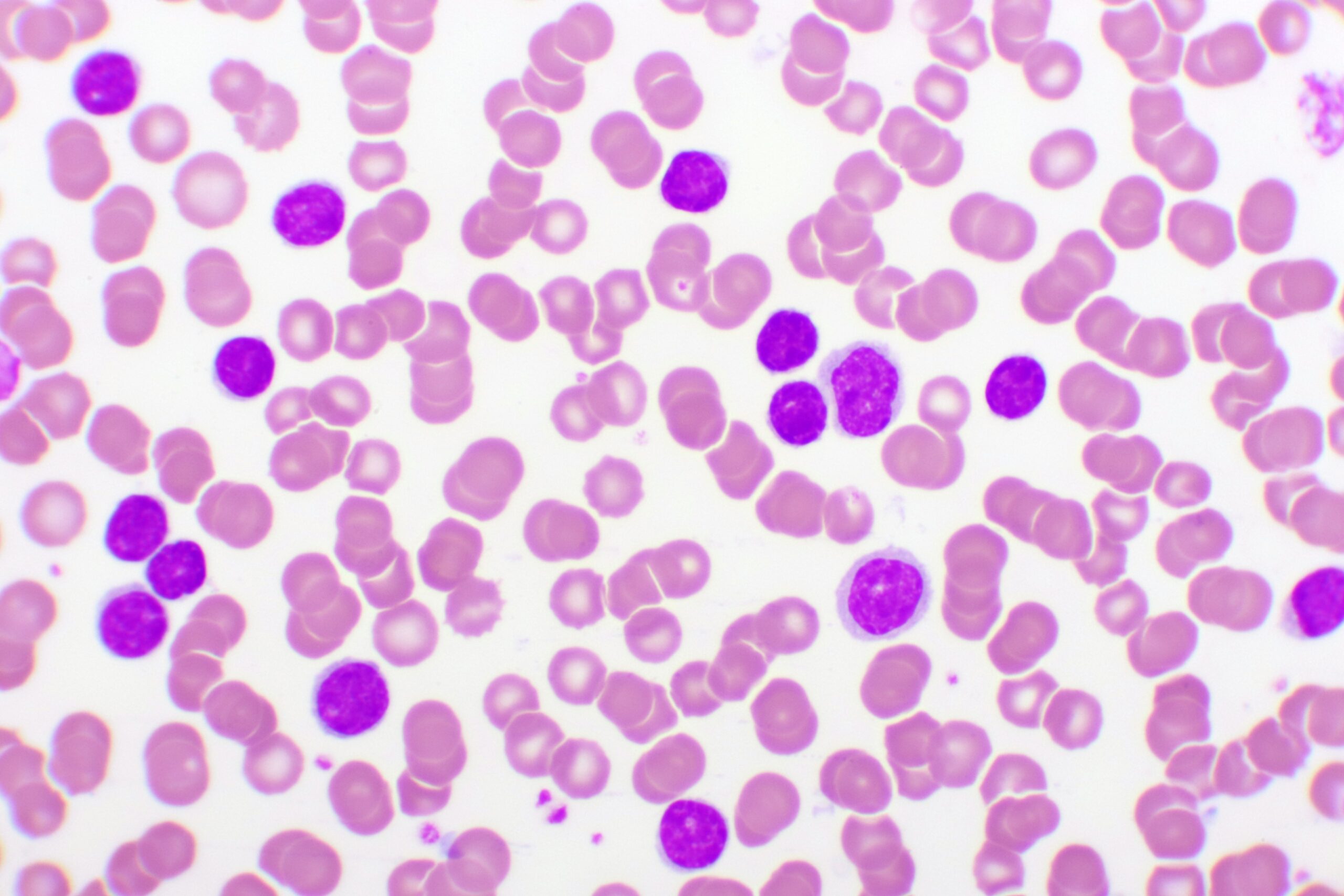
Pirtobrutinib Outperforms Bendamustine-Rituximab in Frontline CLL/SLL
Patients with treatment-naïve chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL) who received pirtobrutinib (Jaypirca; Eli Lilly) monotherapy showed an 80% reduction in progression-free survival (PFS) compared with patients receiving bendamustine plus rituximab (BendaR) in the phase 3 BRUIN CLL-313 (
NCT05023980 ) trial.1Wojciech Jurczak, MD, PhD, head of the department of oncology at Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, presented the data during a late-breaking abstract session at the 67th American Society of Hematology (ASH) Annual Meeting and Exposition.
As targeted therapies have become the standard in CLL, these new data provide insights into pirtobrutinib monotherapy’s potential in the first-line setting, which historically has not included non-covalent BTK inhibitors.2 Following its initial accelerated approval in 2023 for patients with CLL or SLL who had received at least 2 prior therapies, including a BTK inhibitor and a BCL-2 inhibitor, pirtobrutinib was recently granted full approval for patients with CLL or SLL in the relapsed/refractory setting who had previously received a covalent BTK inhibitor.3 The former approval was based on findings from the BRUIN (
NCT03740529 ) and the latter based on the BRUIN-CLL-321 (NCT04666038 ).“While covalent BTK inhibitors have significantly improved outcomes for untreated patients with CLL, at the time of the study design, there were no phase 3 data yet assessing non-covalent BTK inhibitors, especially in the treatment-naïve setting,” Jurczak said during his presentation of the data. He noted that findings from the head-to-head trial BRUIN CLL-314 (
NCT05254743 ), also presented at ASH this year, demonstrated pirtobrutinib’s superiority to the first-generation BTK inhibitor ibrutinib in the first-line setting.4In the open-label, global phase 3 BRUIN CLL-313 trial, 282 patients were randomized 1:1 to receive either pirtobrutinib (n = 141) or BendaR (n = 141), with the opportunity for patients in the BendaR arm to cross over to the pirtobrutinib arm upon confirmed disease progression.1 The primary end point was an independent review committee (IRC)–assessed PFS per International Workshop on Chronic Lymphocytic Leukemia 2018 criteria. The key secondary end point was overall survival (OS), and additional end points included overall response rate (ORR) and safety measures.
At a median follow-up of 28.1 months, the pirtobrutinib arm showed significantly improved IRC-assessed PFS vs the BendaR arm (HR, 0.199; 95% CI, 0.107-0.367; P < .0001), and investigator-assessed PFS was consistent with these findings (HR, 0.186; 95% CI, 0.093-0.371; P < .0001). For patients on pirtobrutinib, the 24-month PFS rate was 93.4% (95% CI, 87.6-96.5) vs 70.7% (95% CI, 61.5-78.1) in the BendaR arm.
Across prespecified, clinically relevant subgroups, IRC-assessed PFS improvement was consistent. This included among patients with mutated and unmutated IGHV (HR for mutated IGHV, 0.293; 95% CI, 0.094-0.910) and HR for unmutated IGHV, 0.172; 95% CI, 0.083-0.357).
The IRC-assessed ORR with pirtobrutinib was 94.3% (95% CI, 89.1%-97.5%) vs 80.9% (95% CI, 73.4%-87%) with BendaR. While the OS data were not mature yet at the interim analysis, the pirtobrutinib cohort demonstrated a notable favorable trend in OS, with an HR of 0.257 (95% CI, 0.070-0.934; P = .0261) compared with BendaR. This was despite 18 of 34 patients with investigator-assessed progressive disease crossing over, representing an effective crossover rate of 52.9%.
Pirtobrutinib also showed a favorable safety profile relative to BendaR, with 40% incidence of grade 3 or higher treatment-emergent adverse effects (TEAEs) vs 67.4% with BendaR. Notably, median treatment duration was 32.3 months for 140 patients receiving pirtobrutinib and 5.6 months for 132 patients receiving BendaR.
Grade 5 TEAEs occurred in 1 patient in the pirtobrutinib arm and 4 patients in the BendaR arm. No grade 5 TEAEs were considered treatment-related in the pirtobrutinib arm, and 1—tumor lysis syndrome—was considered treatment-related in the BendaR arm. A total of 6 (4.3%) patients discontinued treatment with pirtobrutinib due to TEAEs, compared with 20 (15.2%) in the BendaR cohort.
“To conclude, pirtobrutinib had a superior progression-free survival vs bendamustine plus rituximab patients with treatment-naïve chronic lymphocytic leukemia, with one of the largest treatment effects ever observed for a single-agent BTK inhibitor against this competitor,” Jurczak said. “…These data suggest that pirtobrutinib may be considered a potential new standard of care for patients with untreated CLL, especially for the elderly or frail patients who may only receive one line of therapy.”
References
1. Jurczak W, Kwiatek M, Czyz J, et al. Pirtobrutinib vs bendamustine plus rituximab (BR) in patients with CLL/SLL: first results from a randomized phase III study examining a non-covalent BTK inhibitor in untreated patients. Presented at: 67th American Society of Hematology Annual Meeting & Exposition, December 6-9, 2025; Orlando, FL. Abstract LBA-3.
2. Targeted therapy drugs for chronic lymphocytic leukemia (CLL). American Cancer Society. Accessed December 12, 2025.
https://www.cancer.org/cancer/types/chronic-lymphocytic-leukemia/treating/targeted-therapy.html 3. Steinzor P. FDA grants full approval to pirtobrutinib for CLL/SLL. AJMC. December 3, 2025. Accessed December 12, 2025.
https://www.ajmc.com/view/fda-grants-full-approval-to-pirtobrutinib-for-cll-sll 4. Woyach J, Qui L, Grosicki S, et al. Pirtobrutinib vs ibrutinib in treatment-naïve and relapsed/refractory CLL/SLL: results from the first randomized phase III study comparing a non-covalent and covalent BTK inhibitor. Presented at: 67th American Society of Hematology Annual Meeting & Exposition, December 6-9, 2025; Orlando, FL. Poster 683.
Continue Reading
-

Human Cooks Go Head-to-Head With AI-Authored Recipes in New Research
When you roll out chilled sugar cookie dough or top your green bean casserole with crunchy onions this holiday season, you might not consider how much AI went into the recipe — it might be more than you think.
AI involvement in food related activities is becoming increasingly common, according to Stacy Bevan, professional practice associate professor of dietetics at Utah State University. One study reported 74% of people aged 18-24 use AI-powered tools for meal planning, recipe suggestions and grocery shopping. And increasingly, AI is prompted to create the recipes themselves.
But can AI whip up a decent dinner plan? Recently published research from a team in USU’s Department of Nutrition, Dietetics and Food Science tried to find out, pitting human-authored recipes and text against an AI chef. The researchers created two sets of recipe blogs, one authored by students in a food literacy course and another with AI mimicking the students’ style. Researchers then surveyed people’s reactions to the recipe package.
Results showed a growing tolerance for AI-assisted recipe curation, with some important limits, Bevan said. Parts of the process still require a human touch — and a tongue, according to the survey.
AI-generated recipes were rated similarly to the human-written content on several metrics — perceived ease of preparation, use of common ingredients, and time requirements. There was a slight difference in the way participants rated the budget friendliness of recipes, with AI splurging on ingredients more than human authors.
But when people found out who was in the kitchen, the written comments about the experience revealed nuanced reactions. About 43% of participants said that knowing a recipe was AI-generated wouldn’t impact their willingness to try it, but there was serious pause among many based on practical and philosophical concerns.
The most prevalent was that AI wouldn’t have the ability to taste or optimize recipes, perceptions that the recipes might be a bland average of all options and questions about copyright. Many participants in the survey said they preferred human-authored content because AI felt less personal and took away from the “humanness” of working with food and serving a meal.
Many said that they could tell when the text was AI-produced. Some participants said they were fine with AI-generated content as long as the use of AI was disclosed.
“It makes sense that some AI-generated recipes turn out well as they are based on information from existing recipes,” said Katie Kraus, lead author on the research. “But using AI-generated recipes for more complicated dishes is a gamble.”
In a well-written recipe, the narrative and the technical accuracy are important, Bevan said. The narrative around the more technical parts of the process can increase confidence that there is personal experience behind it. It allows readers to know that someone has tested things out and that there is real evidence that it can turn out well, she said. We tend to want to build on other people’s real experience in the kitchen.
Then again, many cooks just jump straight to the recipe.
“My students tell me that they don’t read the narrative anymore,” Bevan said. “The quality of the recipe is more important than the writing these days. Many people, including me, rely more on how many people have reviewed the recipe and how they rate it, or read through a few of the reviews to know people’s real experience.”
The assistance of AI in the kitchen still has tremendous potential. It already does some tasks really well, Bevan said — creating a prep schedule for a big holiday meal, budgeting ingredients on a shopping list and reducing food waste by suggesting meals that use foods already on hand.
And it can be a crack search engine for a good human-written recipe, evaluating thousands and leading you to one that fits your parameters and has high ratings.
But AI can’t replace professional expertise, Bevan said. There are instances where AI has listed inappropriate foods for specific dietary restrictions like diabetic or renal diets. Or given straight up bad advice, like adding non-food items to a recipe.
“It just doesn’t have enough context,” Bevan said. “But it can look good to an inexperienced eye. It is increasingly important to train dietetic students on how to critically engage with and evaluate this kind of content.”
Experienced cooks will be able to identify errors in the recipe and adjust accordingly. This is what Bevan tells her students in her food literacy courses. Classes like this offer a solid foundation in food and nutrition, as well as basics in the kitchen that allow people to translate knowledge about nutrition into actual healthy eating, she said.
“AI is impacting many areas of our students’ lives and future professions,” Kraus said. “We can help them by paying attention to the changes and teaching them to navigate information from a variety of sources, including AI, to create great recipes, understand what they are eating and be healthy.”
Continue Reading
-

Human Cooks Go Head-to-Head With AI-Authored Recipes in New Research
When you roll out chilled sugar cookie dough or top your green bean casserole with crunchy onions this holiday season, you might not consider how much AI went into the recipe — it might be more than you think.
AI involvement in food related activities is becoming increasingly common, according to Stacy Bevan, professional practice associate professor of dietetics at Utah State University. One study reported 74% of people aged 18-24 use AI-powered tools for meal planning, recipe suggestions and grocery shopping. And increasingly, AI is prompted to create the recipes themselves.
But can AI whip up a decent dinner plan? Recently published research from a team in USU’s Department of Nutrition, Dietetics and Food Science tried to find out, pitting human-authored recipes and text against an AI chef. The researchers created two sets of recipe blogs, one authored by students in a food literacy course and another with AI mimicking the students’ style. Researchers then surveyed people’s reactions to the recipe package.
Results showed a growing tolerance for AI-assisted recipe curation, with some important limits, Bevan said. Parts of the process still require a human touch — and a tongue, according to the survey.
AI-generated recipes were rated similarly to the human-written content on several metrics — perceived ease of preparation, use of common ingredients, and time requirements. There was a slight difference in the way participants rated the budget friendliness of recipes, with AI splurging on ingredients more than human authors.
But when people found out who was in the kitchen, the written comments about the experience revealed nuanced reactions. About 43% of participants said that knowing a recipe was AI-generated wouldn’t impact their willingness to try it, but there was serious pause among many based on practical and philosophical concerns.
The most prevalent was that AI wouldn’t have the ability to taste or optimize recipes, perceptions that the recipes might be a bland average of all options and questions about copyright. Many participants in the survey said they preferred human-authored content because AI felt less personal and took away from the “humanness” of working with food and serving a meal.
Many said that they could tell when the text was AI-produced. Some participants said they were fine with AI-generated content as long as the use of AI was disclosed.
“It makes sense that some AI-generated recipes turn out well as they are based on information from existing recipes,” said Katie Kraus, lead author on the research. “But using AI-generated recipes for more complicated dishes is a gamble.”
In a well-written recipe, the narrative and the technical accuracy are important, Bevan said. The narrative around the more technical parts of the process can increase confidence that there is personal experience behind it. It allows readers to know that someone has tested things out and that there is real evidence that it can turn out well, she said. We tend to want to build on other people’s real experience in the kitchen.
Then again, many cooks just jump straight to the recipe.
“My students tell me that they don’t read the narrative anymore,” Bevan said. “The quality of the recipe is more important than the writing these days. Many people, including me, rely more on how many people have reviewed the recipe and how they rate it, or read through a few of the reviews to know people’s real experience.”
The assistance of AI in the kitchen still has tremendous potential. It already does some tasks really well, Bevan said — creating a prep schedule for a big holiday meal, budgeting ingredients on a shopping list and reducing food waste by suggesting meals that use foods already on hand.
And it can be a cracker-jack search engine for a good human-written recipe, evaluating thousands and leading you to one that fits your parameters and has high ratings.
But AI can’t replace professional expertise, Bevan said. There are instances where AI has listed inappropriate foods for specific dietary restrictions like diabetic or renal diets. Or given straight up bad advice, like adding non-food items to a recipe.
“It just doesn’t have enough context,” Bevan said. “But it can look good to an inexperienced eye. It is increasingly important to train dietetic students on how to critically engage with and evaluate this kind of content.”
Experienced cooks will be able to identify errors in the recipe and adjust accordingly. This is what Bevan tells her students in her food literacy courses. Classes like this offer a solid foundation in food and nutrition, as well as basics in the kitchen that allow people to translate knowledge about nutrition into actual healthy eating, she said.
“AI is impacting many areas of our students’ lives and future professions,” Kraus said. “We can help them by paying attention to the changes and teaching them to navigate information from a variety of sources, including AI, to create great recipes, understand what they are eating and be healthy.”
Continue Reading
-

R.I.’s economy in September ‘back to neutral,’ showing positive signs but still in recession, says URI economist – Rhody Today
WHAT: Rhode Island’s economic performance for September was a move back to neutral, however several signs point to the local economy being a bit better than they appear, according to University of Rhode Island economist Leonard Lardaro. September’s CCI value, at 50, matched the marks set in July, April and January. Lardaro suggests there is some underlying momentum that could finally break Rhode Island out of the sub-par range the state found itself in this year. Lardaro’s overall assessment that Rhode Island is in a recession is unchanged. He notes the only question is how long it may last.
For September, six of the 12 economic indicators improved relative to a year ago. However, only two of the five leading economic indicators improved. Total Manufacturing Hours grew at 5%, which Lardaro considers very healthy as both employment and the workweek expanded. Manufacturing Wage also increased by 1.8%—Lardaro calls its recent performance “choppy but strengthening.”
Employment Service Jobs, a leading labor market indicator, rose sharply in September by 3.5%. US Consumer Sentiment again declined by a double-digit rate, down by 21.4%—continuing its consistent monthly declines—Lardaro says.
Retail Sales, a critical economic indicator that has been a strength through almost the entire post-pandemic period, rose by only 2.2%, about half of August’s growth rate. New Claims, which is related to layoffs, increased by 3.5% and sustained its upper trend. Benefits Exhaustion, which indicates long-term unemployment, rose in September by 32.7%, sustaining accelerating increases since June, Lardaro says.
Despite declines in federal employment, Government Employment rose 0.5%. Private Service-Producing Employment, a proxy for service-sector employment, barely rose in September, 0.4%, Lardaro says.
HOW: Use attached information, including summary and charts prepared by Lardaro for news reports. He is available for broadcast and print interviews. Lardaro will be blogging about the new labor data during the coming weeks.
WHEN: Dec. 12, 2025
FOR INFORMATION: Leonard Lardaro, office, 401-874-4128, home, 401-783-9563; James Bessette, URI Department of Communications and Marketing, 401-874-3520, james.bessette@uri.edu.
BACKGROUND: The Current Conditions Index, created by Lardaro, measures the strength of the present economic climate in Rhode Island by following the behavior of 12 indicators.
Continue Reading
-
Tacoma-Pierce County Health Department ensures continued advancement with PHAB reaccreditation – Tacoma-Pierce County Health Department
- Tacoma-Pierce County Health Department ensures continued advancement with PHAB reaccreditation Tacoma-Pierce County Health Department
- Cabell-Huntington Health Department reaccredited by national group herald-dispatch.com
- East Shore District Health Department Celebrates National Public Health Accreditation With Pizza Party Patch
- Chisago County Public Health awarded national accreditation hometownsource.com
- Imperial County Public Health Department earns national accreditation The Desert Review
Continue Reading
-
Nathan Gauvin; Blackridge, LLC; Gray Digital Capital Management USA, LLC; Gray Digital Technologies, LLC
U.S. SECURITIES AND EXCHANGE COMMISSION
Litigation Release No. 26439 / December 12, 2025
Securities and Exchange Commission v. Nathan Gauvin, et al., No. 25-cv-06811 (E.D.N.Y. filed Dec. 10, 2025)
SEC Charges Canadian Citizen and Three Entities with Fraudulent Securities Offerings Targeting Retail Investors
On December 10, 2025, the Securities and Exchange Commission filed charges against Canadian citizen Nathan Gauvin and three entities he controls—Blackridge, LLC, Gray Digital Capital Management USA, LLC, and Gray Digital Technologies, LLC—for orchestrating two fraudulent securities offerings that raised over $18 million from investors across the United States and abroad. Gauvin allegedly misappropriated approximately $6.3 million of investor funds and used fabricated credentials, false performance metrics, and fictitious account statements to lure investors into his schemes.
According to the SEC’s complaint, filed in the U.S. District Court for the Eastern District of New York, Gauvin gained a following on Discord by falsely presenting himself as a successful investment professional managing over a billion dollars in assets through Blackridge, which in reality was a mere shell entity. From September 2022 to November 2024, Gauvin and his entities allegedly raised approximately $18.1 million from investors through an unregistered offering of interests in the “Gray Fund,” a purported diversified investment fund advised by Gray Digital and Gauvin. The complaint alleges that Gauvin and Gray Digital falsely claimed that the Gray Fund generated double-digit monthly returns and held over $78 million in assets, when, in fact, the fund actually had a monthly compounded return of approximately 1.4% and its assets were far lower than claimed. The complaint further alleges that Gauvin misappropriated investor funds to finance a lavish lifestyle, including using hundreds of thousands of dollars for purchases of custom jewelry, luxury concierge services, real estate, and art.
In a second scheme which began in May 2024, Gauvin allegedly offered “seed stock” in Gray Digital Technologies at $30,000 per share, falsely claiming the company had a $60 million valuation and more than $12 million in annual revenue. In reality, the complaint alleges that Gray Digital Technologies had no operations, assets, or revenue. According to the complaint, Gauvin raised at least $60,000 from two retail investors and then ceased communicating with them about this offering.
The SEC’s complaint, filed in the U.S. District Court for the Eastern District of New York, charges Gauvin, Gray Digital Capital Management USA, LLC, and Gray Digital Technologies, LLC with violating Sections 5(a), 5(c), and 17(a) of the Securities Act of 1933; Section 10(b) of the Securities Exchange Act of 1934 and Rule 10b-5 thereunder; and Gauvin and Gray Digital Capital Management USA, LLC with violating Sections 206(1), 206(2), and 206(4) of the Investment Advisers Act of 1940 and Rule 206(4)-8 thereunder. Blackridge, LLC is charged with violating Sections 17(a)(1) and 17(a)(3) of the Securities Act, Section 10(b) of the Exchange Act and Rules 10b-5(a) and (c) thereunder.
The SEC’s investigation was conducted by Clemon Ashley, Ayesha Ahmed, and Matilda Singleton and supervised by Samantha Martin and Jaime Marinaro, and the litigation will be led by Matt Gulde and supervised by Keefe Bernstein, all of the Fort Worth Regional Office.
The SEC appreciates the assistance of the Commodity Futures Trading Commission and the U.S. Attorney’s Office for the Eastern District of New York.
Continue Reading
-
SFA students blend pop culture, business innovation at Swift-Mas Showcase
NACOGDOCHES, Texas –– Students in Stephen F. Austin State University’s Nelson Rusche College of Business stepped into the spotlight during finals week for the Swift-Mas Showcase, an event supported by SFA’s Arnold Center for Entrepreneurship that highlighted student innovation and creative problem-solving throughout the fall semester.
Held outdoors near SFA’s iconic Surfin’ Steve fountain, the showcase served as the final presentation day for “Swiftonomics: The Business of Music and Entrepreneurship,” a course taught by Dr. Amy Mehaffey, lecturer in the Department of Management and Marketing and member of SFA’s Entrepreneurship Faculty Fellows Program.
According to Mehaffey, the course examines Swift not as a celebrity but as an entrepreneur whose mastery of storytelling, values-based branding, market segmentation and audience connection makes her one of the most influential marketers of her generation. Students spent the semester analyzing these strategies and developing their own fully realized product launches inspired by Swift’s approach.
“The best part of this course was watching students realize how capable they are when they’re given creative space,” Mehaffey said. “Taylor Swift was simply an entry point, but what they’re really learning is how to think like marketers, problem-solve and explore life as entrepreneurs.”
During the showcase, attendees explored individual student business concepts featuring prototypes, mockups and visuals that demonstrated how students applied entrepreneurial frameworks to develop innovative solutions for their own brands. Participants described the experience as both energizing and confidence-building.
“This class taught me the most out of all my classes,” said Logan Pantoja, senior marketing major from Weatherford. “I have a whole new understanding and perspective on marketing, and my love for the discipline has only grown stronger. The class strengthened my creative skills and understanding of branding strategy.”
A festive “Swift-Mas” atmosphere elevated the event even further, blending academic rigor with creativity and seasonal celebration. The course’s 43 students representing nine different majors transformed business concepts into practical, audience-ready displays to showcase not only their knowledge but also their ability to think entrepreneurially in a real-world context, according to Mehaffey
“In addition to developing and pitching their own product launches, students engaged in a theory-driven analysis of how public figures navigate brand challenges and scrutiny,” she said. “That assignment pushed them to think critically about reputation management and the strategic decisions behind the headlines. I’m proud to say many students reported leaving the course with strengthened creative problem-solving abilities and a deeper understanding of marketing, branding and entrepreneurship.”
Autumn Nielsen, junior entrepreneurship major from Kingwood, echoed this sentiment.
“We learned how marketing works in the real world and how people respond to different marketing tools,” Nielsen said. “This was so effective because we were talking about a real person and not reading about some concept in a textbook.”
For more information on SFA’s marketing and entrepreneurship academic programs, visit sfasu.edu/mgtmkt. To learn more about the Arnold Center for Entrepreneurship, visit sfasu.edu/ace.
Continue Reading
-

European Union’s AI Omnibus Proposal Offers 10 Key Changes for Companies // Cooley // Global Law Firm
This content is provided for general informational purposes only, and your access or use of the content does not create an attorney-client relationship between you or your organization and Cooley LLP, Cooley (UK) LLP, or any other affiliated practice or entity (collectively referred to as “Cooley”). By accessing this content, you agree that the information provided does not constitute legal or other professional advice. This content is not a substitute for obtaining legal advice from a qualified attorney licensed in your jurisdiction, and you should not act or refrain from acting based on this content. This content may be changed without notice. It is not guaranteed to be complete, correct or up to date, and it may not reflect the most current legal developments. Prior results do not guarantee a similar outcome. Do not send any confidential information to Cooley, as we do not have any duty to keep any information you provide to us confidential. When advising companies, our attorney-client relationship is with the company, not with any individual. This content may have been generated with the assistance of artificial intelligence (Al) in accordance with our Al Principles, may be considered Attorney Advertising and is subject to our legal notices.
Continue Reading
