- Swiss economy contracts slightly in Q3 due to lower pharma exports Reuters
- Swiss economy shrinks significantly in the third quarter bluewin E-Mail
- Switzerland’s Quarterly GDP Down 0.5% in Q3, Final Data Shows MarketScreener
- Swiss GDP contracts -0.5% in Q3, pharma and chemicals lead decline Action Forex
Category: 3. Business
-
Swiss economy contracts slightly in Q3 due to lower pharma exports – Reuters
-

Air Canada Valuation in Focus After New International Route Expansion and Recent Price Volatility
-
Thinking of investing in Air Canada but not sure if the current price reflects its true value? You are not alone. This is a hot topic among investors looking for opportunities.
-
The stock has shown notable volatility, gaining 6.1% over the last week and 5.2% in the last 30 days. However, it is still down 14.7% year-to-date and 23.2% over the past year.
-
Recent discussions around travel demand recovery and evolving industry regulations have added new layers to the Air Canada story. News of expanding international routes and a renewed focus on operational efficiency are fueling debates about the company’s future prospects.
-
Air Canada currently scores 6 out of 6 on our valuation checks, which is a perfect mark. Let us dive into how that score was determined and why a more nuanced perspective on valuation might matter even more by the end of this article.
Find out why Air Canada’s -23.2% return over the last year is lagging behind its peers.
The Discounted Cash Flow (DCF) model estimates a company’s intrinsic value by projecting its future cash flows and discounting them back to today’s value. For Air Canada, this approach uses a 2 Stage Free Cash Flow to Equity method based on analysts’ forecasts for the next five years, with later years extrapolated to gauge long-term trends.
Air Canada’s latest twelve-month Free Cash Flow (FCF) stands at CA$1.71 billion. Analyst estimates indicate this figure could decline in the short run but increase over the next decade. By 2029, projections see annual FCF reaching approximately CA$1.90 billion, with longer-term estimates rising further as industry conditions stabilize and operational efficiencies take effect. All these numbers are presented in Canadian dollars (CA$).
Using these projections, the DCF model estimates Air Canada’s fair value at CA$85.08 per share. This represents a 77.6% premium over the current market price, suggesting the stock is significantly undervalued according to this approach.
Result: UNDERVALUED
Our Discounted Cash Flow (DCF) analysis suggests Air Canada is undervalued by 77.6%. Track this in your watchlist or portfolio, or discover 928 more undervalued stocks based on cash flows.
AC Discounted Cash Flow as at Nov 2025 Head to the Valuation section of our Company Report for more details on how we arrive at this Fair Value for Air Canada.
The price-to-sales (P/S) ratio is a particularly useful valuation measure when analyzing companies that are either coming out of losses or have fluctuating profit margins, as is often the case in the airline industry. Since Air Canada has experienced volatile earnings and recovery is still underway, the P/S ratio provides a straightforward lens to gauge how the market values the company’s revenues, regardless of short-term profitability.
Continue Reading
-
-
Business calls for urgent clarity on EU’s Carbon Border Adjustment Mechanism ahead of January implementation – ICC
In a letter to Commissioner Wopke Hoekstra, ICC stresses that predictable, practical and unambiguous rules are essential to avoid unnecessary trade friction, support investment decisions and preserve the legitimacy of CBAM as a tool to tackle carbon leakage.
The letter highlights nine areas where urgent guidance is required, including:
- Standardised methodologies for calculating and verifying embedded emissions
- Clear rules for default values, benchmarks, and recognition of equivalent carbon pricing regimes
- Alignment with the EU ETS phase-out of free allowances
- Use of existing customs processes and trusted trader frameworks
- A workable approach for the de minimis threshold
- Proportionate treatment of SMEs and developing economies
- A transparent appeals mechanism for non-EU businesses
ICC also calls for the publication of final legislative texts and user-friendly technical guidance well ahead of 2026, alongside strengthened cooperation between the climate and trade communities as CBAM gains prominence in the United Nations Framework Convention on Climate Change (UNFCCC) process.
ICC and its global network stand ready to support the European Commission in ensuring that the final CBAM rules are effective, workable, and fair, and that businesses are equipped to navigate the transition.
Continue Reading
-

Police Kodiaq: RS technology and a spy mode
The development of these purpose-built police specials involved police officers themselves, who worked together with engineers in Mladá Boleslav and drew on extensive experience from the first-generation Kodiaq police fleet. Power now comes from the sporty RS variant and consists of a 2.0 TSI turbocharged petrol engine delivering 195 kW, all-wheel drive, and a DSG automatic transmission. The car is also equipped with specially tuned DCC+ adaptive dampers.
The Škoda Kodiaq will serve both patrol and traffic police units. A new feature is the introduction of 18-inch alloy wheels, replacing the original steel rims with plastic covers. These allow for better cooling of the upgraded more robust braking system, which comes from the seven-seat version of the SUV. Another interesting upgrade is the ability to quickly switch the drivetrain and chassis into sport mode, giving the driver immediate access to full vehicle performance.
Continue Reading
-
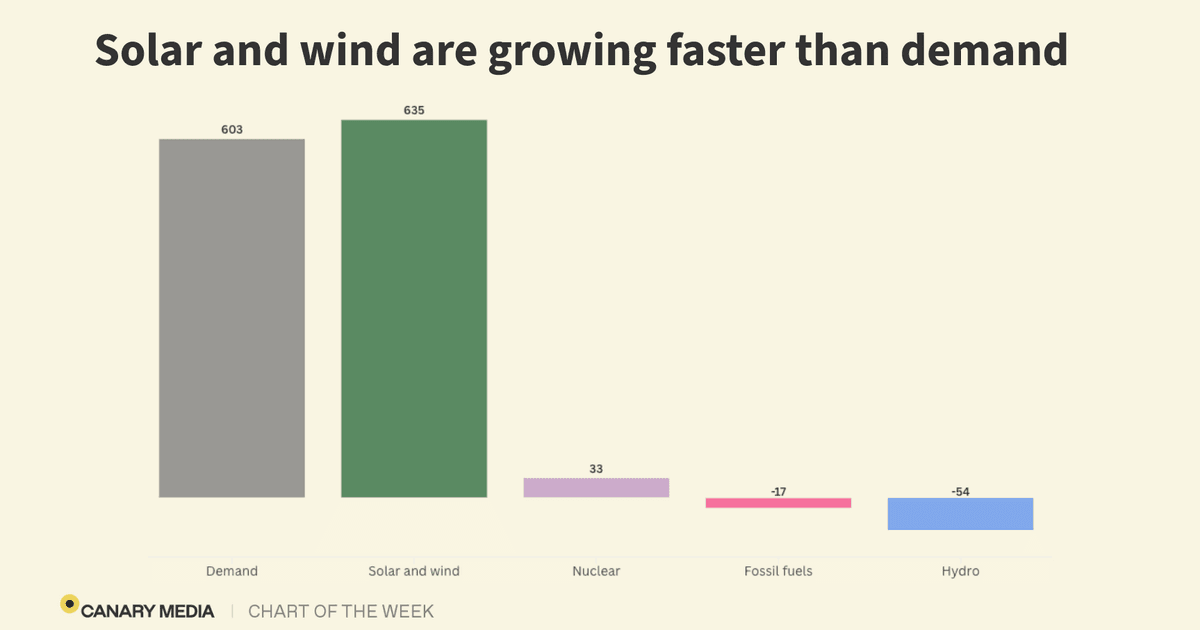
Chart: Solar and wind are meeting — and exceeding —…
Between January and September, the two clean-energy sources grew fast enough to more than offset all new demand worldwide, according to data from energy research firm Ember.
Power demand rose by 603 terawatt-hours compared to that same time period last year. Solar met nearly all that new demand on its own, increasing by 498 TWh. Wind generation, meanwhile, climbed by 137 TWh.
What happens when clean energy not only meets but exceeds new power demand? We start to burn less fossil fuels. At least a little less: Through Q3, fossil-fuel generation dropped by 17 TWh, compared to the first three quarters of 2024. This trend is expected to continue through the end of the year. Ember forecasts that fossil-fuel generation will have experienced no notable growth in 2025 — something that hasn’t happened since the height of the Covid-19 pandemic.
It’s unclear whether this flatlining marks the beginning of the end for fossil-fueled electricity or whether it’s just a pause before another surge in dirty power. The answer will more or less be determined by what grows faster: electricity demand or renewable energy.
Common consensus is that the world’s appetite for electricity will expand rapidly in the coming years. The planet is warming and driving increased use of air conditioning. AI developers are building massive power-hungry data centers. Cars, homes, and factories are being electrified. That all adds up: The International Energy Agency expects power demand to rise by a staggering 40% over the next decade.
Meanwhile, it’s almost not worth considering long-term forecasts about the growth of clean energy, given how inaccurate they’ve been in the past. Analysts have consistently underestimated solar, in particular.
For the global power sector to truly decarbonize, carbon-free energy needs to not only keep pace with electricity demand but far outrun it. Let’s hope solar continues to overperform.
Continue Reading
-

MHI Publishes “MHI REPORT 2025” and “SUSTAINABILITY DATABOOK 2025”

Tokyo, November 28, 2025 – Mitsubishi Heavy Industries, Ltd. (MHI) published its integrated report, “MHI REPORT 2025”, which provides a balance of financial and non-financial information of MHI Group and its “SUSTAINABILITY DATABOOK 2025” (the Databook), an annual report summarizing non-financial information of the Group.
“MHI REPORT 2025” provides explanations centering on our new corporate strategy, Innovative Total Optimization (ITO) established by CEO Eisaku Ito, who took the office in April. In a message from the President and CEO at the beginning, CEO Ito outlines his management approach to achieve Group-Wide Optimization and Reach Expansion to unlock growth potential and establish a virtuous cycle of high profitability and growth investments by creating new value. Next, in a message from the CFO, CFO Hiroshi Nishio shares his perspective on the financial strategy that supports medium- to long-term growth to meet the capital market’s expectations of the Group, from perspectives such as financial discipline, resource allocation, and portfolio management.
Two feature articles are included. The topic of the first feature is at the forefront of our future growth areas. A message from CSO Masayuki Suematsu explains our growth strategy, centering on the initiatives for growth areas established in the 2024 Medium-Term Business Plan. In a roundtable discussion on the data center business, the feature discusses the future outlook of the market, the Group’s strengths, and its eagerness to expand this business. The topic of the second feature is the technology platform supporting MHI Group. In a message from the CTO, CTO Tomoaki Omura shares his own mission to transform our technology platform and lay the groundwork for businesses that can underpin growth in the future, in addition to contributing to the ongoing business operations of the Group. The feature introduces the Shared Technology Framework, which is designed to serve as a Group-wide hub for technology.
The latter half of the report contains an article covering a roundtable discussion on the Company’s governance system. Three outside directors exchange opinions reflecting on the ten years since the Company transitioned to an Audit and Supervisory Committee structure in 2015. The functions of the Nomination and Remuneration Committee and principles on CEO succession are also covered.
The Databook provides the public with information on the progress being achieved by MHI Group in its sustainability management strategy, with content divided into sustainability management, the environment, society, and governance, along with detailed performance data.
The 2025 edition of the Databook includes expanded coverage of the Company’s initiatives related to the circular economy.(Note1) Also included are information on the certification of “Wadaoki Forest,” a forested area cultivated within Mihara Machinery Works in Hiroshima Prefecture as a “Nationally Certified Sustainably Managed Natural Site” by the Ministry of the Environment, and the “Strategy Map for Well-being and Health Management” developed to promote employee health and well-being.(Note2)
MHI Group aims to contribute to the resolution of the issues facing the world with our diverse technologies, thereby providing value to customers and society while also enhancing our corporate value. Going forward, we will continue to clearly communicate this mission to a broad range of stakeholders.
- 1 A circular economy is an economic system that aims to efficiently circulate resources to promote a sustainable society along with economic growth.
- 2Well-being is a concept signifying a state in which individual rights and self-fulfillment are guaranteed, and physical, mental, and social conditions are good.
Continue Reading
-
European shares set for monthly gains, helped by Fed rate cut bets – Reuters
- European shares set for monthly gains, helped by Fed rate cut bets Reuters
- STOXX 600 ends steady after multiple-session rally Business Recorder
- EMEA Morning Briefing: Fed’s Rate Trajectory in Focus 富途牛牛
- Europe opens mostly flat with data in focus breakingthenews.net
- Fed Cut Hopes Lift European Stocks As AI Fears Cool Finimize
Continue Reading
-

Porsche Leipzig plant receives Automotive Lean Production Award
1. All information offered on Porsche Newsroom, including but not limited to, texts, images, audio and video documents, are subject to copyright or other legislation for the protection of intellectual property. They are intended exclusively for use by journalists as a source for their own media reporting and are not intended for commercial use, in particular for advertising purposes. It is not permitted to pass on texts, images, audio or video data to unauthorised third parties.
2. Use of Newsroom content for book projects (or similar commercial use) is not permitted, particular with regards to images. Any potential usage must be approved beforehand by Dr. Ing. h.c. F. Porsche AG. To discuss licencing requests for book projects please email: archiv@porsche.de
3. All logos and trademarks mentioned on Porsche Newsroom are trademarks of Dr. Ing. h.c. F. Porsche AG (hereinafter: Porsche AG), unless otherwise stated.
4. All contents of Porsche Newsroom are carefully researched and compiled. Nevertheless, the information may contain errors or inaccuracies. Porsche AG does not accept any liability with respect to the results that may be achived through the use of the information, in particular with respect to accuracy, up-to-dateness and completeness.
5. Insofar as Porsche Newsroom provides information concerning vehicles, the data refers to the German market. Statements concerning standard equipment and statutory, legal and tax regulations and repercussion are valid for the Federal Public of Germany only.
6. With respect to the use of Porsche Newsroom, technical faults such as, delays to news transmission, cannot be ruled out. Porsche AG does not accept any liability for any resulting damage.
7. Insofar as Porsche Newsroom provides links to the internet sites of third parties, Porsche AG does not accept any responsibility for the content of the linked sites. On using the links, the user leaves the Porsche AG information products.
8. In agreeing to these rights of use, the user shall be obliged to refrain from any improper use of Porsche Newsroom.
9. In the event of improper use, Porsche AG reserves the right to block access to Porsche Newsroom.
10. Should one or more provisions of these terms and conditions be or become invalid, this shall not affect the validity of the remaining provisions.
Continue Reading
-

I3 Instrument Support Facility virtual event: “Find your regional match: online speed networking for stakeholders”
The I3 Instrument, funded by the ERDF, supports interregional collaboration in shared smart specialisation areas, fostering innovation-driven industrial transitions, and cross-regional value chains. As part of the Work Programme 2025–2027, the new Strand 2b call is open and will remain open until 19 March 2026.
This call represents a significant opportunity for both more developed and transition regions to strengthen innovation capacity, and for less developed regions to connect with broader European ecosystems. However, finding suitable partners with complementary strengths and shared priorities remains a challenge.
To address this, the 15th I3 Instrument Support Facility virtual event will provide a structured online matchmaking format to help participants build new partnerships for future I3 Instrument project proposals.
Participants will:
- Meet potential partners from other EU regions considering engagement in I3 Instrument projects;
- Explore complementarities and shared smart specialisation areas;
- Foster early-stage discussions that may lead to concrete I3 Instrument consortia or future cooperation opportunities.
The format will consist of three networking rounds in small breakout groups (3–4 participants), each lasting about 20 minutes, supported by light thematic prompts to guide discussion. An optional fourth networking round will be offered at the end for further exchanges.
Continue Reading
-

US retailers are about to see if Black Friday benefits from a holiday halo effect
NEW YORK — NEW YORK (AP) — Black Friday bargains no longer tempt people to leave Thanksgiving tables for midnight mall runs. Brawls in store aisles over toys and TVs with limited-time discounts are spectacles of holidays past. Online shopping and retailers launching discounts weeks before the turkey feast subdued that kind of fervor.
But the sales event still has enough enthusiasts to make the day after Thanksgiving the one when U.S. stores get the most shoppers coming in the door. For that reason, Black Friday still rules as the unofficial start of the holiday shopping season.
This year’s kickoff comes as consumer confidence in the U.S. economy fell this month in the aftermath of the federal government shutdown, weak hiring and stubborn inflation, according to a report The Conference Board issued Tuesday.
Many retail executives have reported customers becoming more discerning and increasingly focused on deals while at the same time remaining willing to splash out for important occasions like the start of the school year and the winter holidays, creating a halo effect.
“Consumers have been saying the economy is terrible while continuing to spend for years now, so the outlook is probably better than they are telling us,” Bill Adams, the chief economist at Comerica Bank, said this week of shoppers’ moods heading into Black Friday. “But business surveys also report consumers are being more sensitive to prices and selective in spending.”
While planning for the holidays in the spring and summer, retail companies were wrestling with the volatility of President Donald Trump ’s wide-ranging tariffs on imported goods. Many accelerated shipments of some merchandise before the tariffs took effect or decided to absorb some of the import tax costs instead of raising prices for customers.
Market research firm Circana said that 40% of all general merchandise sold in September saw a price increase of at least 5% compared with the first four months of the year.
Toys, baby products, housewares, and team sports equipment were among the hardest hit categories. For example, 83% of toys sold in September saw an increase of at least 5%, Circana said. Industry group The Toy Association says nearly 80% of the toys sold in the U.S. are made in China, a country the Trump administration hit with especially high tariffs at various points this year.
Still, analysts and mall executives cited solid momentum heading into Black Friday week. At the Mall of America in Bloomington, Minnesota, foot traffic in recent weeks surpassed the numbers from pre-pandemic 2019, said Jill Renslow, the mall’s chief business development and marketing officer.
“We’re seeing a very positive start to the holiday season,” Renslow said. “The last few Saturdays in November have been very strong.”
The growth in online sales also has been robust so far. From Nov. 1 to Nov. 23, consumers spent $79.7 billion, according to web tracking and analysis platform Adobe Analytics. That represented a gain of 7.5% from a year earlier and was bigger than Adobe’s 5.3% growth forecast for the season.
Mastercard SpendingPulse, which tracks spending across all payment methods, predicted a 3.6% increase in holiday sales from Nov. 1 through Dec. 24. That compares with a 4.1% increase last year.
“Clearly, there’s uncertainty,” Mastercard Chief Economist Michelle Meyer said. “Clearly, consumers feel on edge. But at the moment, it doesn’t seem like it’s changing how they are showing up for this season.”
According to Adobe Analytics, Thanksgiving Day was the best time to shop online to get the deepest discount on sporting goods. But Black Friday will be the best time to buy TVs, toys and appliances online.
Cyber Monday, however, should be the best time to buy apparel and computers. Apparel discounts peaked at 12.2% off the suggested manufacturer’s price between Nov. 1 and Nov. 23 but are expected to hit 25% off on Cyber Monday, Adobe said.
Continue Reading