Early Tuesday morning, a total lunar eclipse will occur.
A total lunar eclipse, often nicknamed a “blood moon” because of its reddish hue, takes place when the moon passes through Earth’s shadow…

Early Tuesday morning, a total lunar eclipse will occur.
A total lunar eclipse, often nicknamed a “blood moon” because of its reddish hue, takes place when the moon passes through Earth’s shadow…
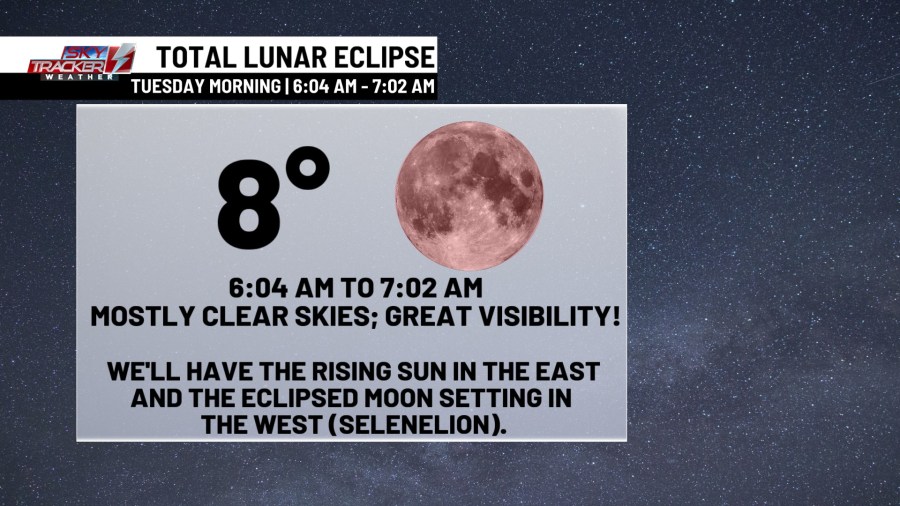
Make it a point to get up early tomorrow morning. Here are more details about Tuesday’s total lunar eclipse!
Timing: Totality will last about 58 minutes from 6:04 AM to 7:02 AM. That’s when the moon is set to glow a reddish-brownish hue.
Continue Reading

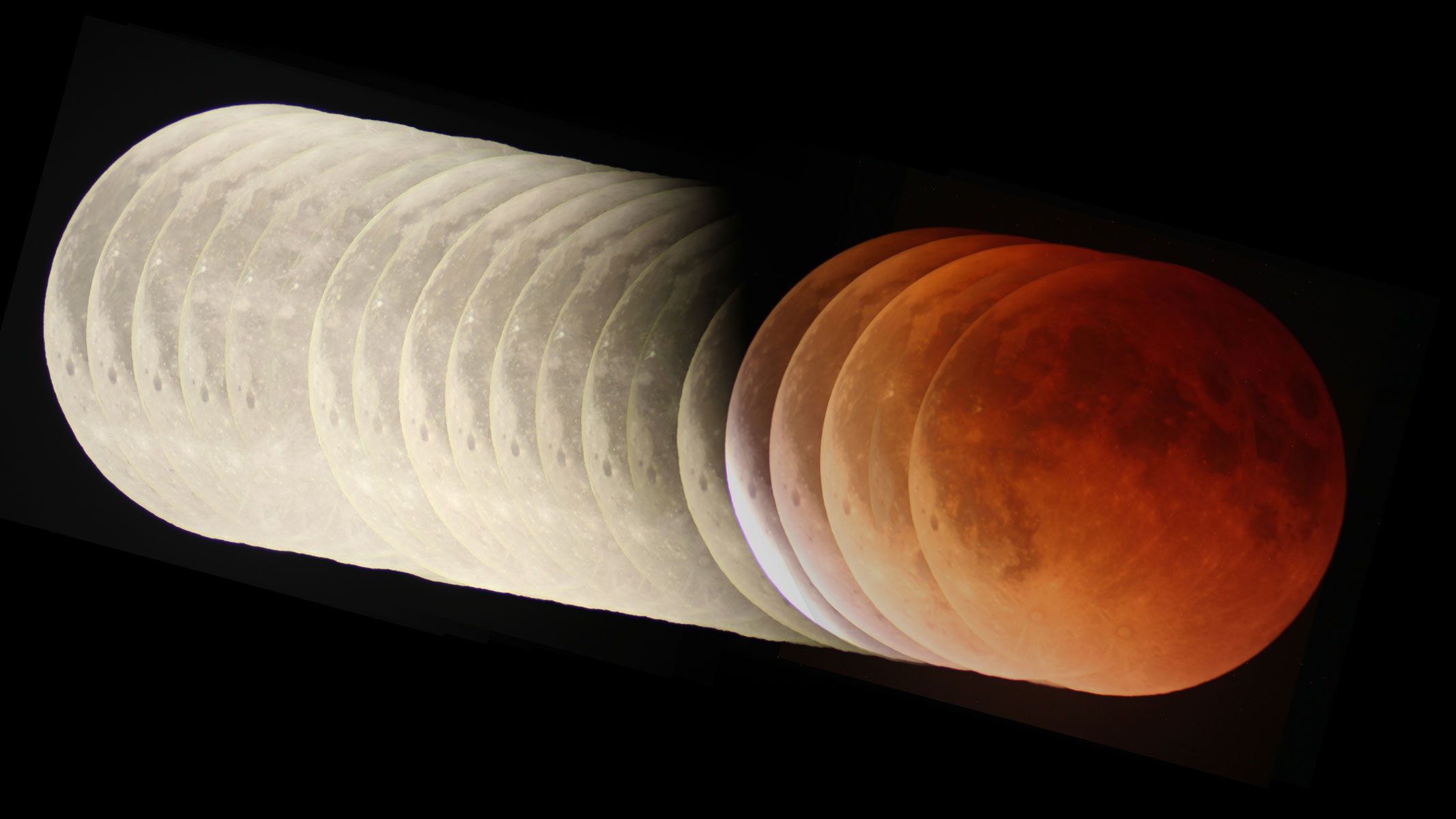
On March 3, 2026, the full Worm Moon will slip into Earth’s shadow and turn a strange copper-reddish color for 58 minutes. To the naked eye, it will be a beautiful sight, with the full moon’s light extinguished as a “blood moon” sits in a…

On March 3, billions of people across the Americas, Asia and Oceania will witness a blood moon total lunar eclipse as the sun, Earth and moon align, laying bare the orbital mechanics of the solar system in spectacular fashion.
The eclipse occurs…

Early land plants may have begun reshaping Earth”s surface much earlier than previously thought — pushing the timeline back by about 30 million years, according to a new study.
The findings, resulting from research by…

North America, Australia and New Zealand will be treated to a rare total lunar eclipse on Tuesday known as a “blood moon”.
As the full moon dips into the planet’s shadow it will change colour to a “deep and coppery red”, says…


SpaceX is all set to launch a Falcon 9 rocket early Sunday…