This article was originally published at The Conversation. The publication contributed the article to Space.com’s Expert Voices: Op-Ed & Insights.
If you’ve seen illustrations or models of the solar system, maybe you noticed that all the…

This article was originally published at The Conversation. The publication contributed the article to Space.com’s Expert Voices: Op-Ed & Insights.
If you’ve seen illustrations or models of the solar system, maybe you noticed that all the…

On Jan. 11, 2026, I watched anxiously at the tightly controlled Vandenberg Space Force Base in California as an awe-inspiring SpaceX Falcon 9 rocket carried NASA’s new exoplanet telescope, Pandora, into orbit.
Exoplanets are worlds that orbit…


Scientists from Institute of Geology and Geophysics (IGG), Chinese Academy of Scienceshave now provided new insights using high-precision potassium isotope analysis of samples collected from the Moon’s far side…
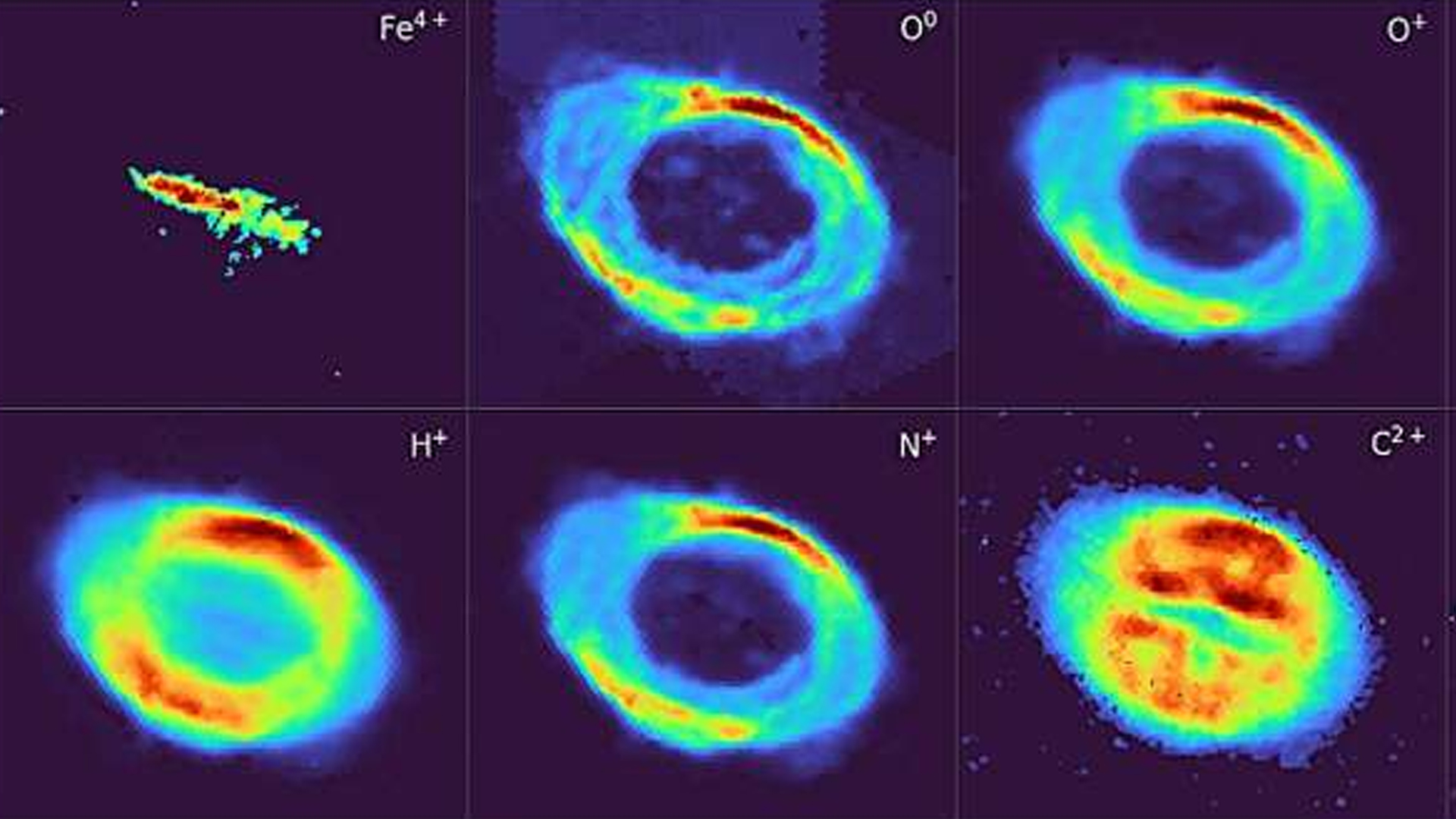
Have you ever seen a cloud made of iron? You may not believe it, but deep inside a nebula that astronomers have studied for centuries, researchers have uncovered a massive bar-shaped cloud made almost entirely of iron atoms.
This glowing…

Elon Musk embraced a video praising the starship, Space X’s 120-meter reusable…

The night sky could soon lose some of its natural darkness if a controversial space project…