Satellites must be able to withstand the violent forces of lift-off and survive in the harsh space environment. To make sure they can function reliably when in orbit, every satellite and its components undergo extensive testing before…
Category: 7. Science
-

Tokyo targets the two infinities – CERN Courier
Across the universe About 200 researchers gathered in Tokyo to discuss physics at the largest and smallest scales. Credit:… Continue Reading
-
Europes crop droughts to get worse even as rain increases
Europe and western North America will experience more frequent and severe crop droughts as the planet warms, even in places where yearly rainfall increases.
Scientists from the University of Reading studied how climate…
Continue Reading
-

New Model Fails to Explain Near-Death Experiences, Scientists Say
Newswise — An ambitious effort to create a neurophysiological paradigm to explain near-death experiences has failed to capture many fascinating and often perplexing aspects of people’s brushes with death, top…
Continue Reading
-

Wolverhampton University opens AI humanities innovation hub
Attendees at the launch will hear from several professors talking about subjects including AI, technology, chatbots and algorithms used to analyse complex data.
“As higher education evolves, the arts and humanities are embracing digital and…
Continue Reading
-
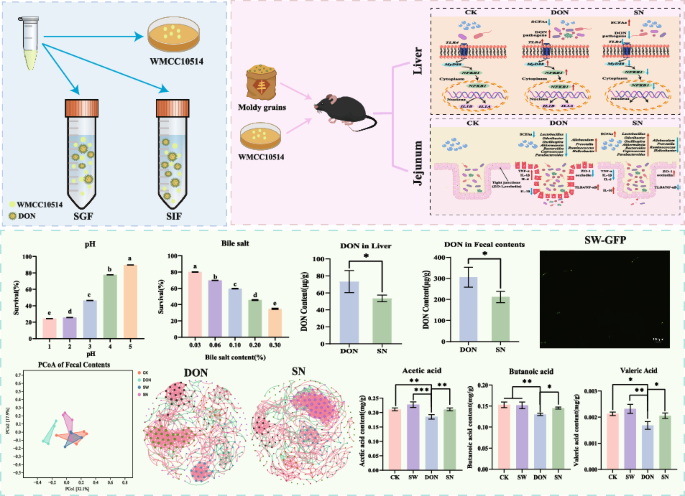
Bacillus velezensis mitigates deoxynivalenol-induced intestinal inflammation and liver injury via modulating the gut microbiota
Pestka, J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 3, 323–347 (2010).
Ndiaye, S. et al. Current review of mycotoxin…
Continue Reading
-

Tomaso Poggio on his quest for theories to explain the fundamental learning abilities of brains and machines
In this episode of “Brain Inspired,” Paul Middlebrooks talks with Tomaso Poggio, director of the Center for Biological and Computational Learning and the Center for Brains, Minds, and Machines, both at the Massachusetts Institute…
Continue Reading
-

Can we use bees as a model of intelligent alien life to develop interstellar communication?
Humans have always been fascinated with space. We frequently question whether we’re alone in the universe. If not, what does intelligent life look like? And how would aliens communicate?
The possibility of extraterrestrial life is grounded in…
Continue Reading
-

Study explores how biological and environmental systems regulate body weight
Pennington Biomedical researchers recently investigated the systems of the body that regulate weight, exploring whether our bodies defend an established weight target or if our bodies operate within a broader range of…
Continue Reading