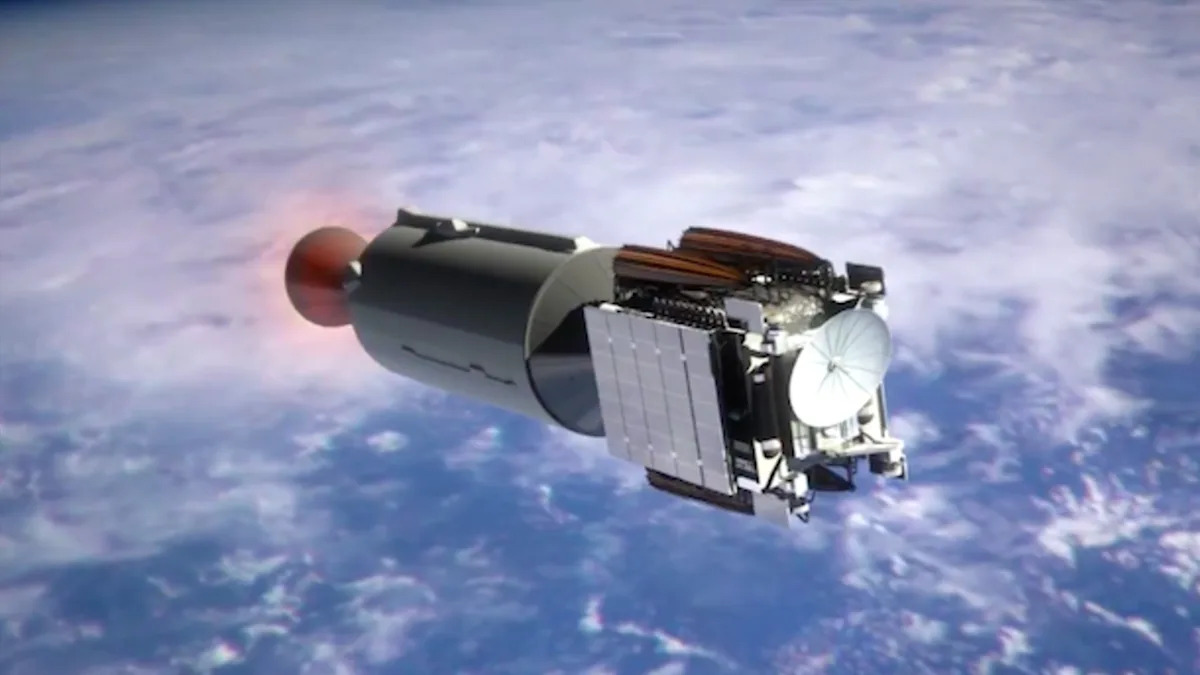
A new study of Earth’s upper atmosphere has discovered spikes in lithium ion levels following the return of a Falcon 9 rocket upper stage, which broke up on reentry. With SpaceX planning to rapidly increase its number of orbiting satellites by
Category: 7. Science
-

Meet the Gigamaser—the Brightest Microwave Laser Ever Spotted in Deep Space
Space is full of odd light sources astronomers don’t quite understand, like double supernovas, weird blue flashes, random Venn diagrams, and more. And just when we thought we’d seen it all, there’s a new addition to the…
Continue Reading
-

50 year quest ends with creation of silicon aromatic once thought impossible
Major scientific advances often require patience, and this discovery is a prime example. After nearly 50 years of theory and repeated failed attempts by research groups around the world, David Scheschkewitz, Professor of General and Inorganic…
Continue Reading
-

The Last Mystery of Antarctica’s ‘Blood Falls’ Has Finally Been Solved
There is a corner of Antarctica that looks like something out of a David Cronenberg movie. It’s located in the dry valleys of McMurdo, an immense frozen desert where, periodically, a jet of crimson liquid suddenly gushes from the dazzling white…
Continue Reading
-
CINEMA mission will explore auroras and Earth's mysterious magnetotail – Phys.org
- CINEMA mission will explore auroras and Earth’s mysterious magnetotail Phys.org
- NASA Fires Twin Rockets to “CT Scan” the Northern Lights and Map Hidden Auroral Currents The Debrief
- OTD in space – February 17: NASA launches 5 THEMIS…
Continue Reading
-

The Legal Void of the Asteroid Gold Rush
Asteroid mining companies are finally getting off the ground, and that is raising some concerns about the impact those activities will have on the space environment. A new paper published in Acta Astronautica from Anna Marie Brenna of…
Continue Reading
-

Daring Space Mission Would Catch Up With 3I/ATLAS and Intercept It
Interstellar object 3I/ATLAS provided scientists with an exceptionally rare opportunity to study the nature of other planetary systems beyond our own. It was first discovered in July of last year, heading straight past the Sun and making its
Continue Reading
-

Can solar storms trigger earthquakes? Scientists propose surprising link
Scientists at Kyoto University have developed a theoretical model examining whether disturbances in the ionosphere could apply electrostatic forces deep within the Earth’s crust. Under certain conditions, these forces might contribute to the…
Continue Reading
-

Starlight warped in the fabric of spacetime could help us find hidden black holes dancing together
Two supermassive black holes on a dizzying death spiral could soon become visible to astronomers after researchers worked out how, while rotating around each other, these dark, massive behemoths could gravitationally lens the stars behind…
Continue Reading