CAPE TOWN, Jan. 7 (Xinhua) — The Square Kilometer Array Observatory (SKAO) announced on Wednesday that the growing SKA-Mid telescope array in South Africa has achieved “first fringes” using two of its dishes, which demonstrates its operation…
Category: 7. Science
-
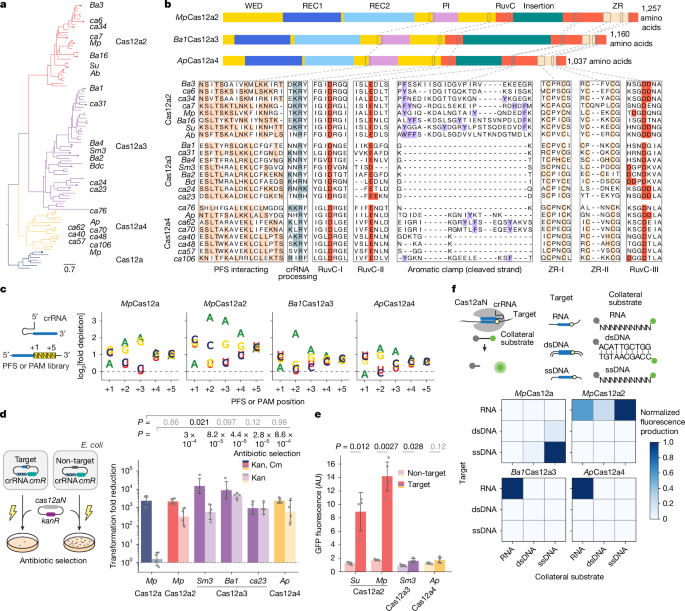
RNA-triggered Cas12a3 cleaves tRNA tails to execute bacterial immunity
Orthologue identification, phylogenetic analysis and sequence alignment
We used previously identified Cas12a2 protein sequences27 as queries for tBLASTn and BLASTp searches in the NCBI databases (https://www.ncbi.nlm.nih.gov) and the JGI…
Continue Reading
-
SKA-Mid telescope in South Africa achieves first fringes milestone-Xinhua
CAPE TOWN, Jan. 7 (Xinhua) — The Square Kilometer Array Observatory (SKAO) announced on Wednesday that the growing SKA-Mid telescope array in South Africa has achieved “first fringes” using two of its dishes, which demonstrates its operation…
Continue Reading
-
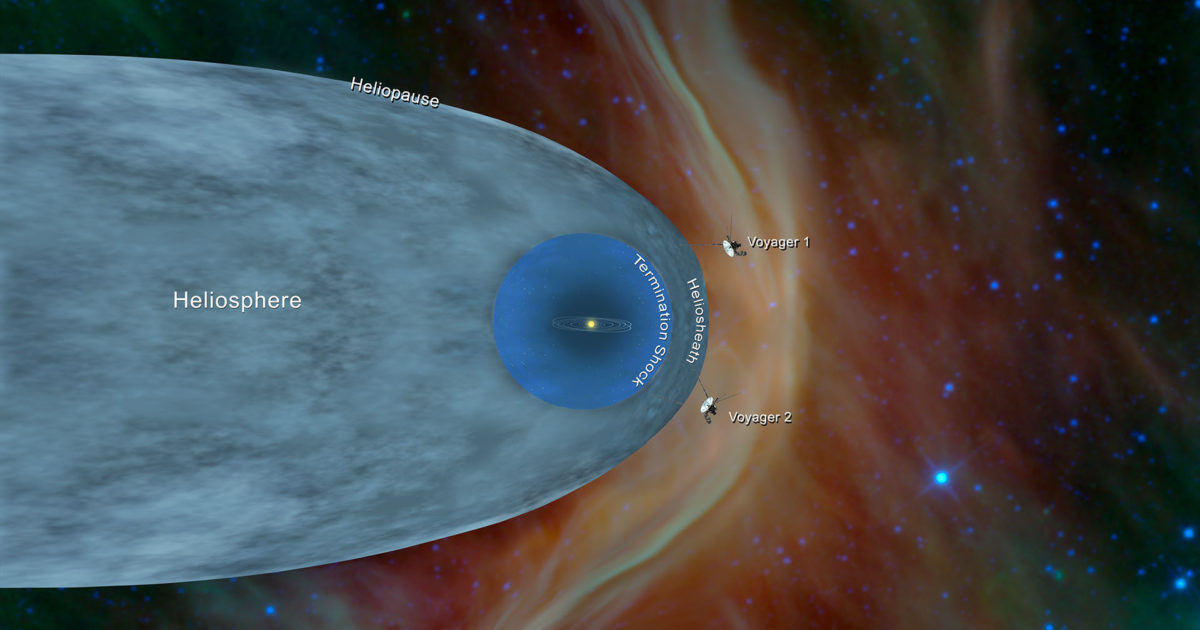
Voyager and the heliopause: Exploring where…
Sarah Al-Ahmed:
We’re exploring where the Sun gives way to the stars, this week on Planetary Radio. I’m Sarah Al-Ahmed of The Planetary Society with more of the human adventure across our solar system and beyond.Nearly 50 years after launch, the…
Continue Reading
-
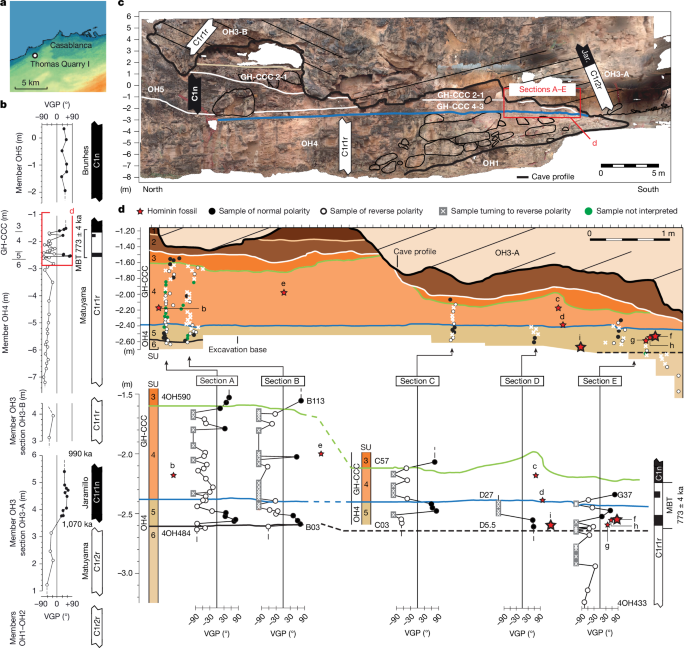
Early hominins from Morocco basal to the Homo sapiens lineage
Meyer, M. et al. Nuclear DNA sequences from the Middle Pleistocene Sima de los Huesos hominins. Nature 531, 504–507 (2016).
Google Scholar
Duval, M. et al. The…
Continue Reading
-
Risks young chimps take as they swing through the trees underscore role of protective parenting in humans
Adolescents are known for risky behavior, with teenagers in the U.S. more likely than younger children to die from injury. But what’s responsible for this uptick in risk-taking around puberty?
Our new observations of physical risk-taking…
Continue Reading
-
Moroccan Cave Fossils Yield a Possible Missing Link in Human Evolution – The New York Times
- Moroccan Cave Fossils Yield a Possible Missing Link in Human Evolution The New York Times
- Moroccan Cave Fossils Capture a Crossroads in Modern Human Evolution ScienceAlert
- We have a fossil closer to our split with Neanderthals and Denisovans Ars…
Continue Reading
-

Most planets in our galaxy are born ‘bloated’, new study suggests
Astronomers may have uncovered how the most common types of planets in our Galaxy grow, a new study has revealed. The discovery confirms astronomers’ theories that these planets start as ‘bloated’ babies but quickly lose much of their thick…
Continue Reading
-

Synthetic skin based on octopus biology promises uses in robotics
Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
Scientists have unveiled a synthetic skin inspired by octopus camouflage that is capable of changing colour and texture,…
Continue Reading
-
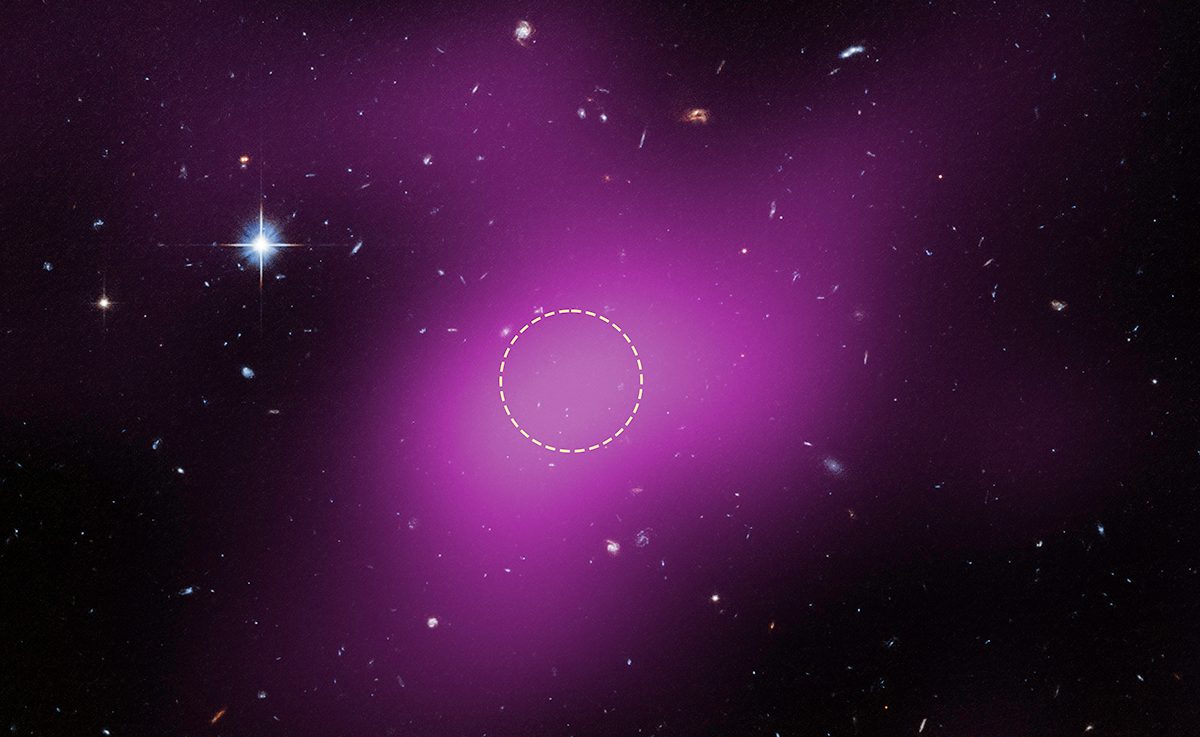
Scientists have found a new kind of object in deep space. And it could help solve one of the Universe’s biggest mysteries
Astronomers using the Hubble Space Telescope have identified an entirely new kind of object in the Universe.
This strange object is a starless, gas-rich cloud that’s dominated by dark matter, and is thought to be a remnant left over from the…
Continue Reading