Paleontologists in Brazil have identified a previously unknown species of somphospondylan sauropod dinosaur with European affinities, hinting at ancient migration routes that once linked two continents now separated by the Atlantic Ocean.
An…

Paleontologists in Brazil have identified a previously unknown species of somphospondylan sauropod dinosaur with European affinities, hinting at ancient migration routes that once linked two continents now separated by the Atlantic Ocean.
An…
BYLINE: Lisa Potter, research communications specialist, University of Utah communications
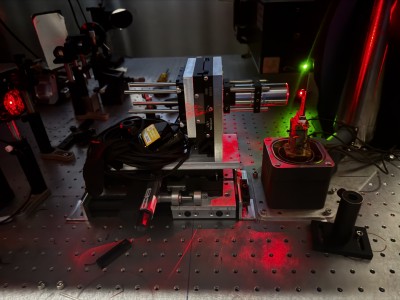
Newswise — To keep up with today’s computing needs, researchers mine the quantum realm to…

Key takeaways
ChatGPT Health launched in January 2026 as OpenAI’s consumer health tool, reaching millions of users. Here, we conducted a structured stress test of triage recommendations using 60 clinician-authored vignettes across 21 clinical domains under…
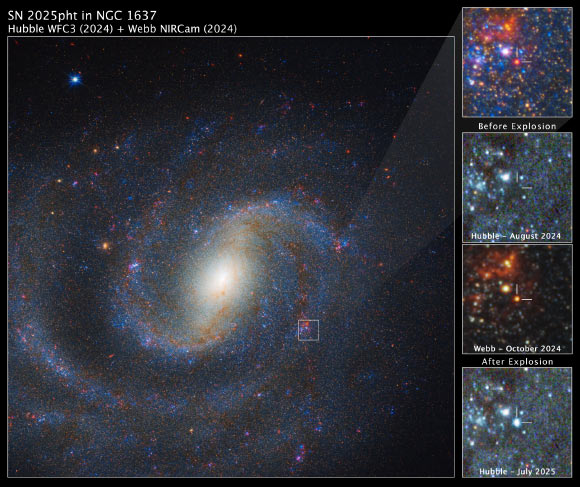
Using the NASA/ESA/CSA James Webb Space Telescope, astronomers have for the first time identified the progenitor of a nearby supernova — a red supergiant star cloaked in thick, dust-rich shrouds that made it invisible to previous…
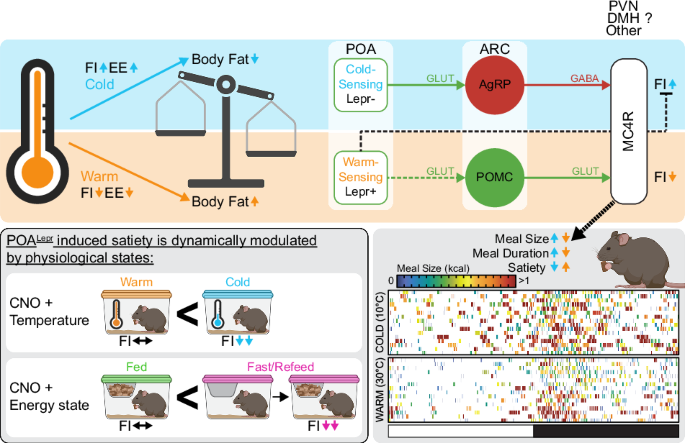
Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1207–R1228 (2011).
Yu, S., Francois, M., Huesing, C. &…

The study was funded by the National Science Foundation and led by Grace Johnston, who conducted the research as a student. Johnston was recruited into Argueso’s lab as an undergraduate biology student and wrote the paper as her…

Well, I guess we jinxed it.
One day after NASA completed a wet dress rehearsal for the Artemis 2 mission with no major issues, the Space Launch System (SLS) rocket started acting up again. This time, it wasn’t a hydrogen leak….
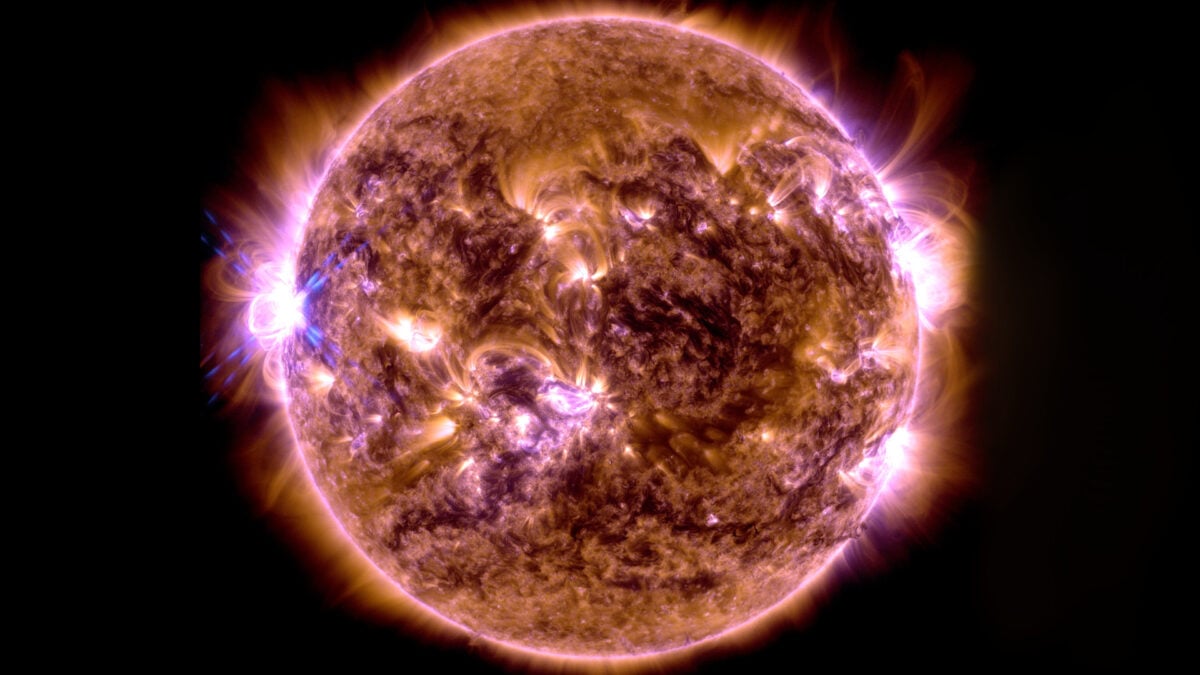
Earthquakes are one of many natural phenomena that, despite technological advances, we’ve yet to predict in advance. Researchers in Japan—a country frequently hit by devastating earthquakes—propose we look for an…