There are some logical things we know to be true: working out is tiring. And being tired helps you fall asleep. But exercise before bed can sometimes have the opposite effect. I have personal experience with this: At one point I was attending a
Category: 7. Science
-
Earliest, hottest galaxy cluster gas on record challenges cosmological models – Phys.org
- Earliest, hottest galaxy cluster gas on record challenges cosmological models Phys.org
- Earliest, hottest galaxy cluster gas on record could change cosmological models UBC Science
- Impossibly Hot Object Discovered 1.4 Billion Years After The Big…
Continue Reading
-
"Too Strong to Be Real": Astronomers Stunned by Boiling Gas in the Early Universe – SciTechDaily
- “Too Strong to Be Real”: Astronomers Stunned by Boiling Gas in the Early Universe SciTechDaily
- Earliest, hottest galaxy cluster gas on record could change cosmological models UBC Science
- Impossibly Hot Object Discovered 1.4 Billion Years After…
Continue Reading
-
Mars Curiosity Rover Observes Sulfur Crystals – astrobiology.com
- Mars Curiosity Rover Observes Sulfur Crystals astrobiology.com
- Mars Curiosity rover cracked open a rock and finds an element that should not be there Earth.com
- Curiosity drove over a rock on Mars, accidentally breaking it, and what appeared…
Continue Reading
-

Impossibly Hot Object Discovered 1.4 Billion Years After The Big Bang
A ‘shadow’ cast on the faint, leftover glow of the Big Bang has revealed a giant object in the early Universe that defies our predictions of how the Universe should evolve.
It’s a galaxy cluster named SPT2349-56. Spotted a mere 1.4 billion years…
Continue Reading
-

NASA SMD ROSES-25 F.6 Science Activation: Corrections of Requirements for Budget Submission
NASA Astrobiology Program…
Continue Reading
-

Astrobites at AAS 247: Welcome!
This week, AAS Nova and Astrobites are attending the American Astronomical Society (AAS) winter meeting in Phoenix, AZ.
AAS Nova Editors Kerry Hensley and Susanna Kohler and AAS Media Fellow Lexi Gault will join Astrobites Media Intern…
Continue Reading
-

My 101-Year-Old Grandma Had Many Friends: Her Secrets to Build Community
Whenever I visited my grandmother, Laura, at her senior-living community, I felt like a celebrity. She seemed to know everyone, and was so loved — the folks there were all too happy to tell me so.
We often played…
Continue Reading
-

Scientists Warn: Long-term Environmental Data Eroding
A new Special Report published in the journal BioScience warns that long-term ecological and evolutionary research faces severe threats from lack of recurring funding and governmental/institutional support, to data manipulation and…
Continue Reading
-
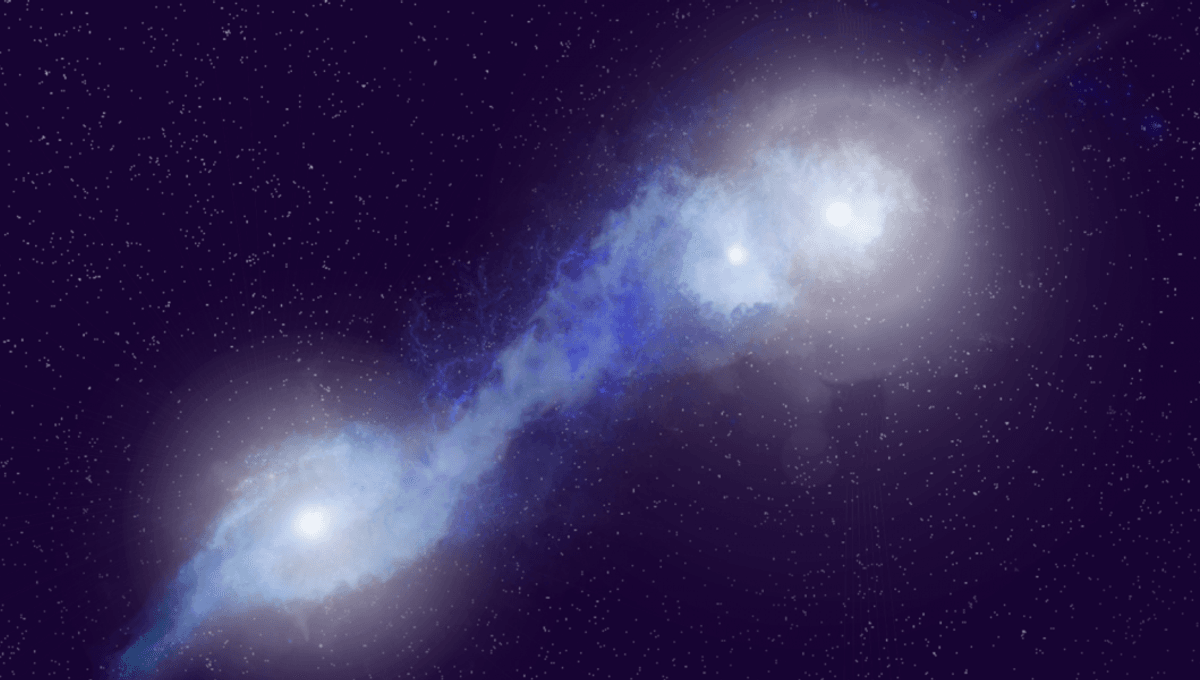
First Ever Radio System With Three Active Supermassive Black Holes Discovered
Galaxies might be separated by hundreds of thousands of light-years at the very least, but they do occasionally merge. During those collisions, the supermassive black holes that sit at the center of those galaxies can become active, entering a…
Continue Reading
