After the excitement of three visible comets in 2025, another may put on a show in late April 2026. However, although the comet, known as C/2025 R3 (PanSTARRS), could become bright enough to be seen with the naked eye, it could just as easily…
Category: 7. Science
-

Multi-Material Microrobot Grabs And Releases Cells
Original story from the International Journal of Extreme Manufacturing.
A tiny microrobot enables the performance of precise movements, including grasping, delivering and releasing particles or cells, with applications in medicine,…
Continue Reading
-

Only 2 planets shine in January’s night sky to the naked eye — here’s where to look
TOP TELESCOPE PICK:
(Image credit: Amazon) Want to see the visible planets in the night sky? The Celestron NexStar 4SE is ideal for beginners wanting quality, reliable and quick views of celestial objects. For a more in-depth look at our
Continue Reading
-
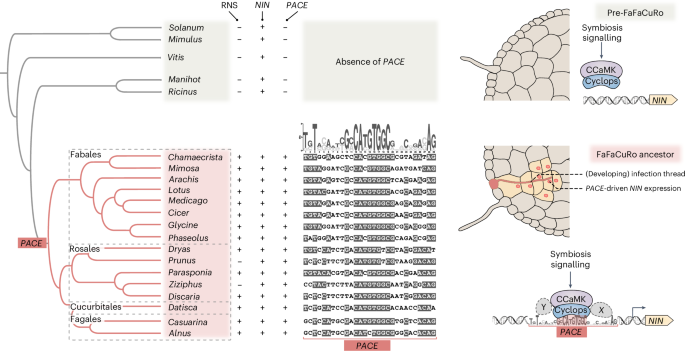
A novel cis-element enabled bacterial uptake by plant cells
LeBauer, D. & Treseder, K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 (2008).
Google Scholar
Continue Reading
-

China’s ‘artificial sun’ experiment finds way to break fusion plasma density limit
HEFEI — Researchers have identified a method to surpass the plasma density limit in experiments with the Experimental Advanced Superconducting Tokamak (EAST), known as the “artificial sun,” providing a crucial physical…
Continue Reading
-
Superradiant spins show teamwork at the quantum scale
When quantum particles work together, they can produce signals far stronger than any one particle could generate alone. This collective phenomenon, called superradiance, is a powerful example of…
Continue Reading
-

2026 is going to be one of the best years ever for fans of the full Moon. Here’s why
Are you a fan of the full Moon? From supermoons to lunar eclipses and even just plain old, regular full Moons, people all over the world love the sight of a beautiful, bright Moon in the night sky.
Unlike any other planet in our Solar System,…
Continue Reading
-

Fires May Emit More Air Pollution Than Previously Estimated
As fires burn the landscape, they spew airborne gases and particles, though their impact on air pollution might be underestimated. A study in ACS’ Environmental Science & Technology reports that, around the world, wildfires and…
Continue Reading
-

Aria for chemist who wants to tackle key challege of our age | News
The Advanced Research and Invention Agency (Aria), a funding body dedicated to high-risk, high-reward research, hit the headlines last year, when it awarded £57 million in funding for a range of projects centred on controversial approaches…
Continue Reading
-
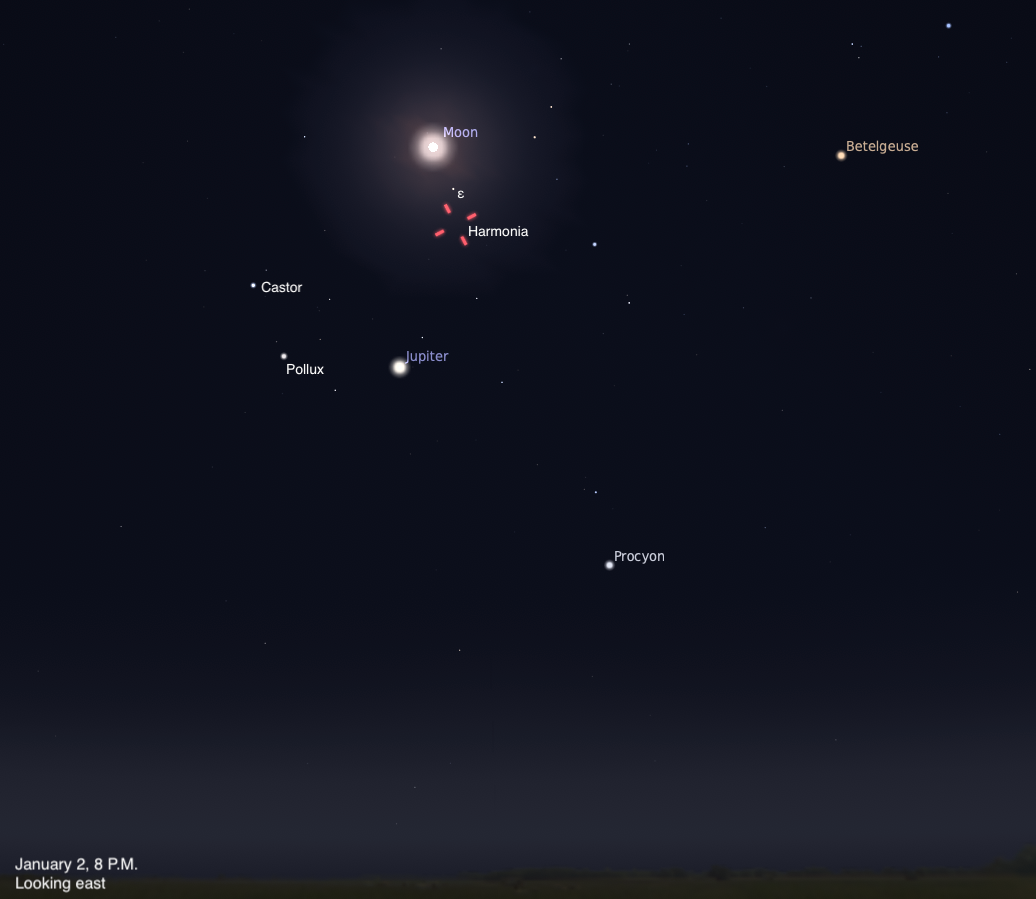
The Sky Today on Friday, January 2: Harmonia at opposition
Asteroid Harmonia reaches opposition in Gemini the Twins, near the bright planet Jupiter in the evening sky.
Asteroid Harmonia reaches…
Continue Reading
