Space is unforgiving to electronics. Beyond Earth’s protective magnetic field, satellites are bombarded by cosmic rays and high-energy particles that slowly chip away at delicate circuits.
Over time, these invisible strikes can corrupt…
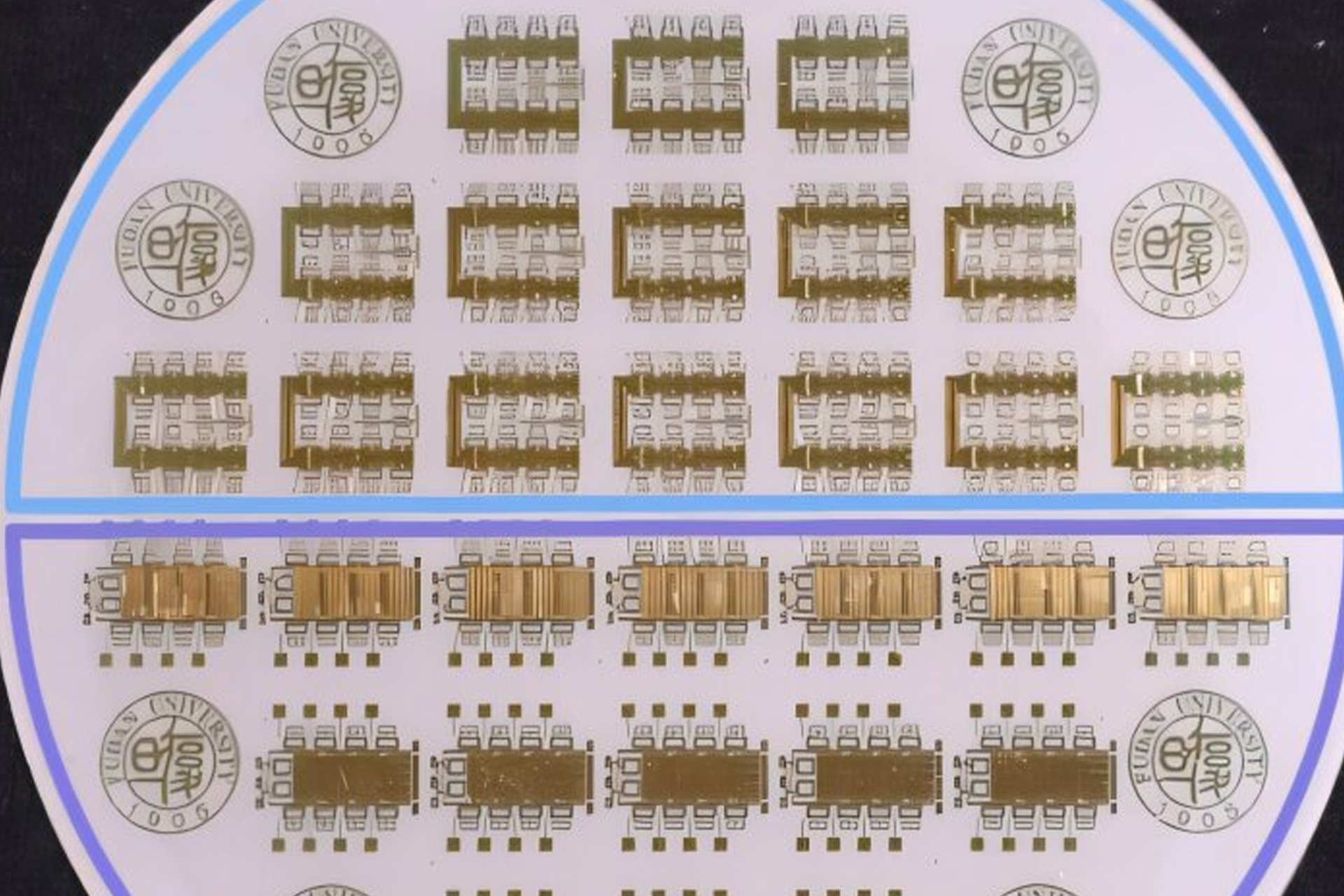
Space is unforgiving to electronics. Beyond Earth’s protective magnetic field, satellites are bombarded by cosmic rays and high-energy particles that slowly chip away at delicate circuits.
Over time, these invisible strikes can corrupt…

Planetary nebula NGC 3242 in Hydra is popularly known as the Ghost of Jupiter. You’ll find this glowing ball of gas in the sky late tonight.
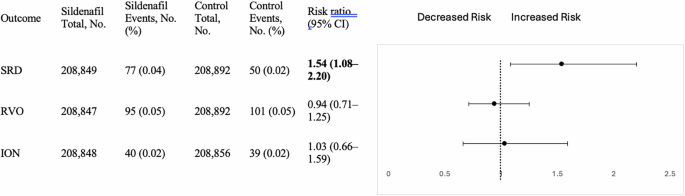
Phosphodiesterase type 5 inhibitors (PDE5i) are widely used for erectile dysfunction. Case reports and claims-based cohort studies have suggested associations between PDE5i use and ocular adverse events, including serous retinal detachment (SRD),…

For the first time, astronomers have charted the vertical structure of Uranus’s upper atmosphere, revealing how temperature and electrically charged particles change with altitude across the planet. An international research team used the James…

For the first time, astronomers have charted the vertical structure of Uranus’s upper atmosphere, revealing how temperature and electrically charged particles change with altitude across the planet. An international research team used the James…
Bowyer, T. W. et al. Detection and analysis of xenon isotopes for the comprehensive nuclear-test-ban treaty international monitoring system. J. Environ. Radioact. 59(2), 139–151 (2002).
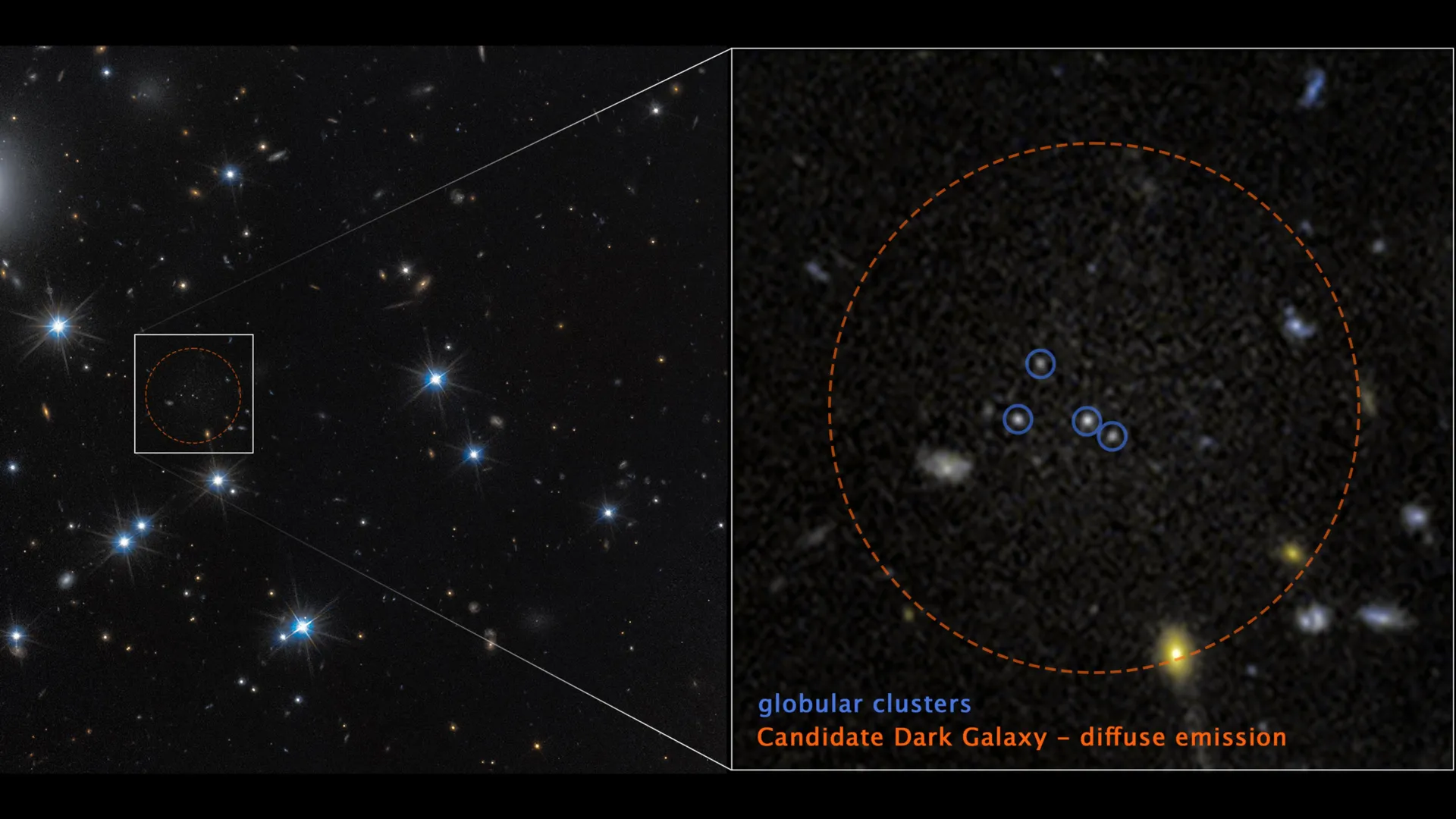
Most galaxies blaze with billions of stars, lighting up the universe across vast distances. But a small and unusual group barely glows at all. These are low-surface-brightness galaxies, systems so faint they are difficult to detect and so sparse…


A cross-disciplinary research team from Tsinghua…

BEIJING, Feb. 20 (Xinhua) — Chinese researchers have developed an artificial intelligence (AI) model for astronomical imaging that significantly enhances scientists’ ability to peer into the deepest reaches of the…