Before a space telescope ever reaches orbit, and long after satellites are up there, NASA has another way to do frontier science: high-altitude scientific balloons. These helium balloons can loft instruments to roughly 120,000 feet (about 36.6…
Category: 7. Science
-

Hubble captured the dramatic aftermath of colliding space rocks in a nearby planetary system
Far away in the universe, something surprising happened that no astronomer saw coming. NASA’s Hubble Space Telescope spotted the glowing remains of two space rocks crashing together in a nearby planetary system, a rare cosmic event that looked…
Continue Reading
-

New Eyes on One of the Planet’s Largest Submarine Landslides
When it comes to landslides, some of our planet’s largest have occurred underwater. But out of sight shouldn’t mean out of mind—submarine landslides can be both damaging and dangerous.
Researchers have now mapped the Stad Slide, an…
Continue Reading
-

New plant fiber-based artificial synapse holds memory longer
Researchers are building a computer chip-like device that mimics the brain, and eventually dissolves into the dirt after use.
The Ulsan National Institute of Science and Technology (UNIST) in South Korea has developed a fully biodegradable…
Continue Reading
-
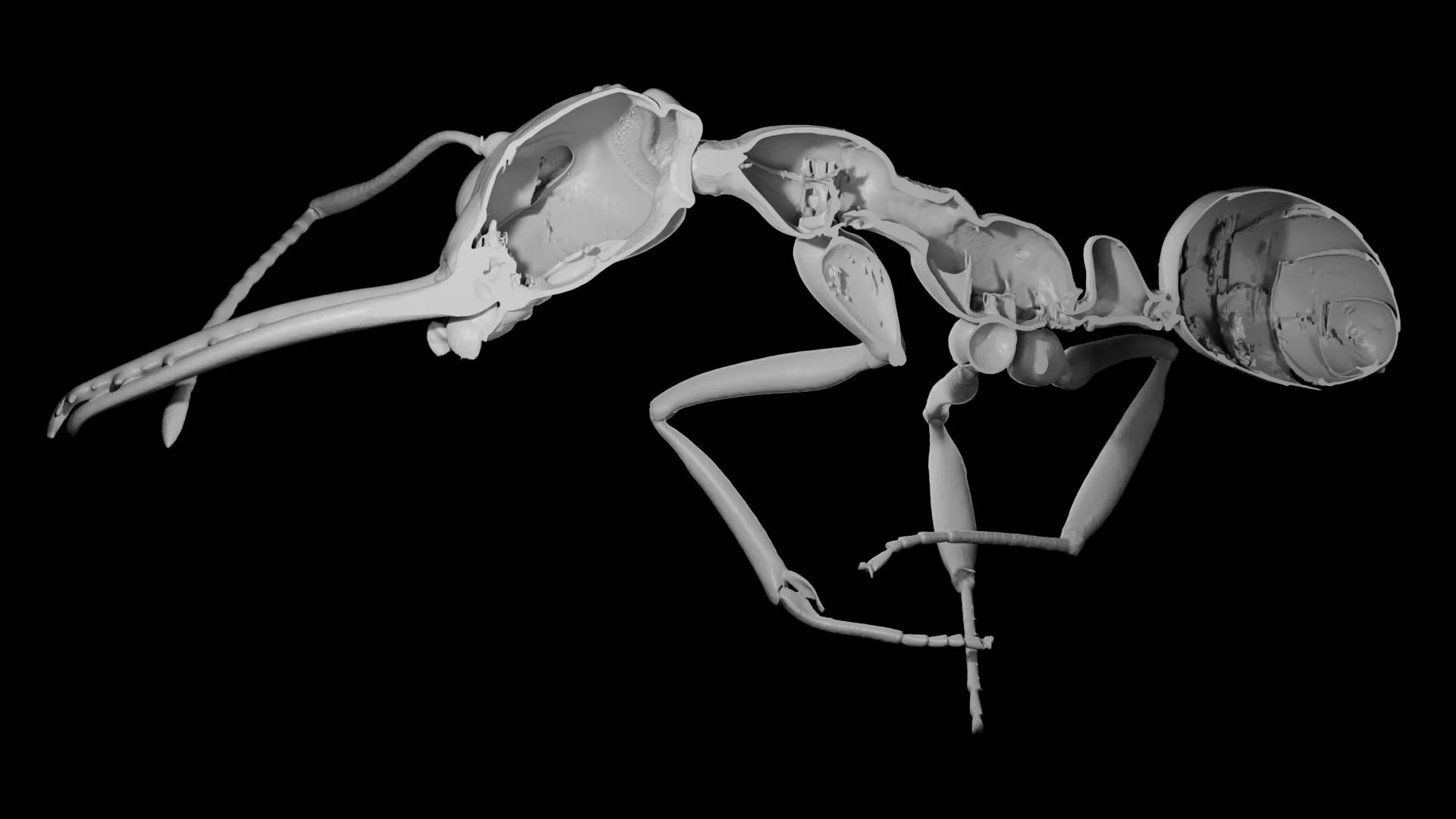
Why evolution rewarded ants that sacrificed protection
The question is playful and unrealistic, but it points to a serious idea: the tension between quantity and quality. New research suggests this same tradeoff has shaped evolution, especially in the rise of complex animal societies.
How ants…
Continue Reading
-

Faraday’s Enigma Of Premelted Ice Finally Explained After 166 Years
Water can be liquid at temperatures far below freezing under several conditions; perhaps the most surprising is as a thin layer on the surface of ice. The discovery of this fact dates at least to 1859, but there have only been partial…
Continue Reading
-
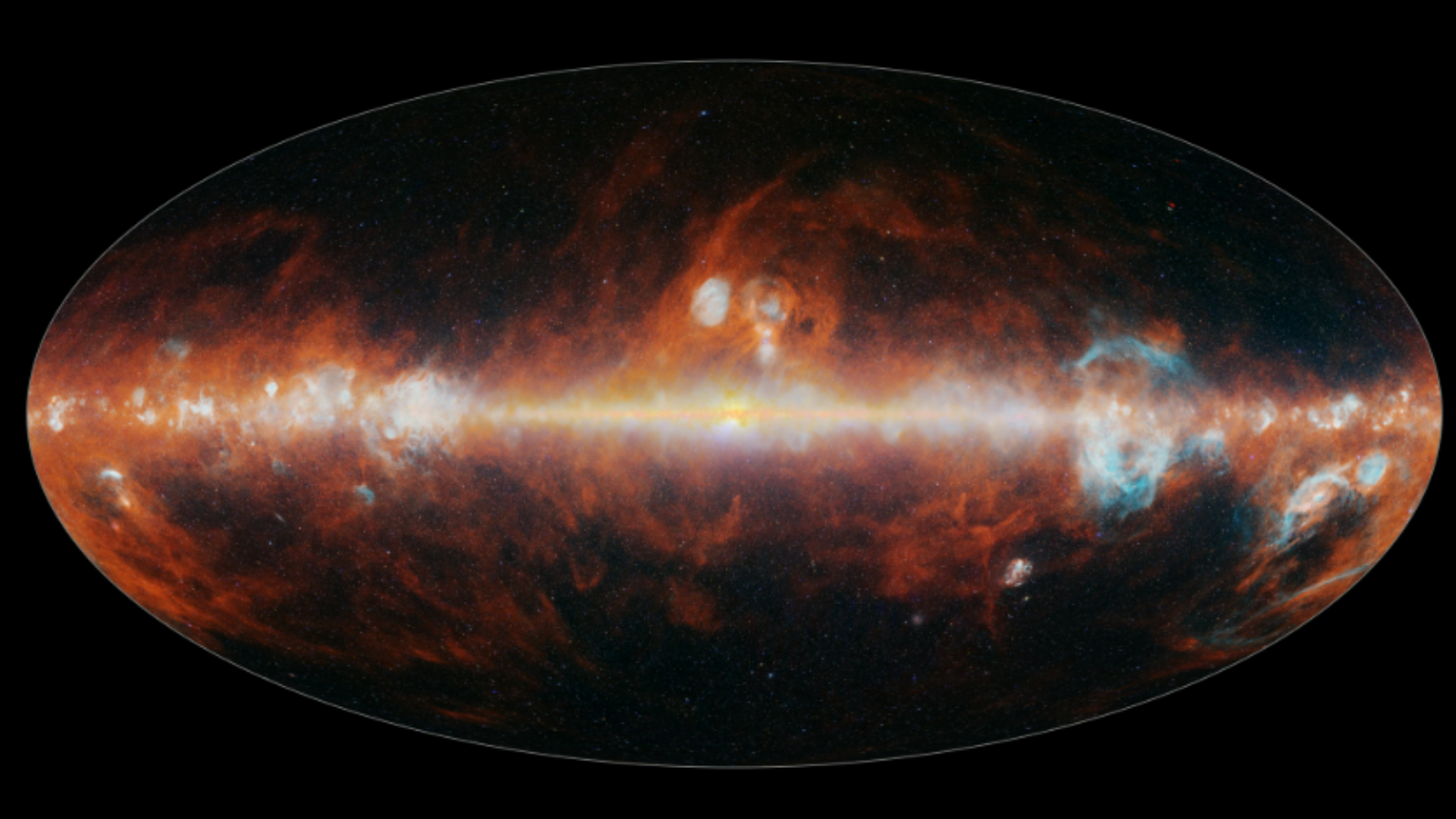
NASA’s SPHEREx telescope completes its 1st cosmic map of the entire sky and it’s stunning!
NASA’s SPHEREx observatory has completed its first map of the entire sky over Earth, and it is incredible.
Continue Reading
-

What really happens near black holes? New study reveals startling findings
The researchers in a recent breakthrough have successfully modelled…
Continue Reading
-

ESA – Euclid’s galaxy garland
Galaxy NGC 646 sparkles like a cosmic holiday garland in this new image from the European Space Agency’s Euclid space telescope.
This large barred spiral galaxy is located in the constellation Hydrus and was discovered in 1834 by the…
Continue Reading

