Sarah Al-Ahmed:
We’re celebrating the 30th anniversary of the Galileo Mission’s orbital insertion around Jupiter this week on Planetary Radio. I’m Sarah Al-Ahmed of The Planetary Society with more of the human adventure across our solar system…
Category: 7. Science
-

Galileo at 30: How a mission transformed our…
-

Planned launch of 500,000 satellites may destroy Hubble’s mission
Simulations suggest that satellite streaks could spoil about one in three Hubble images, even when the telescope stays above Earth’s weather.
A team of researchers modeled plans that could put about 560,000 satellites aloft by the 2030s.
Continue Reading
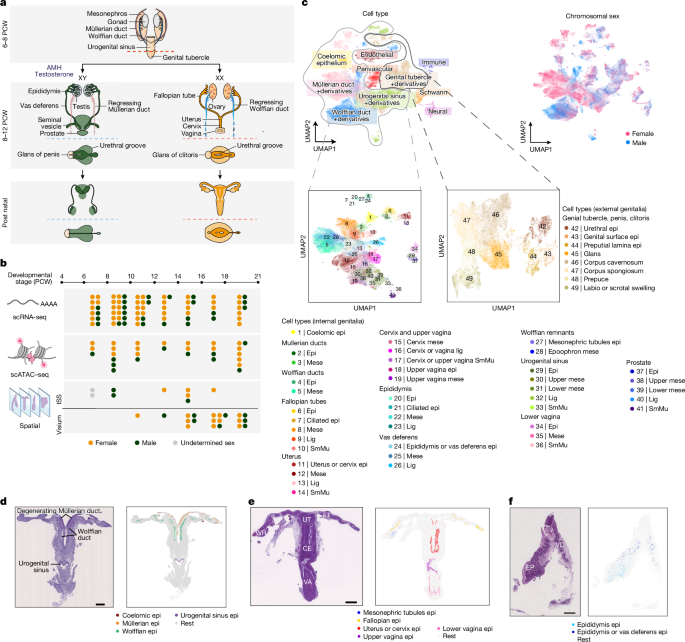
Spatiotemporal cellular map of the developing human reproductive tract
Samples
Fetuses were obtained after voluntary terminations of pregnancy, which were performed either via medical or surgical procedures. The termination methods used did not compromise the integrity or morphology of the fetuses analysed in this…
Continue Reading
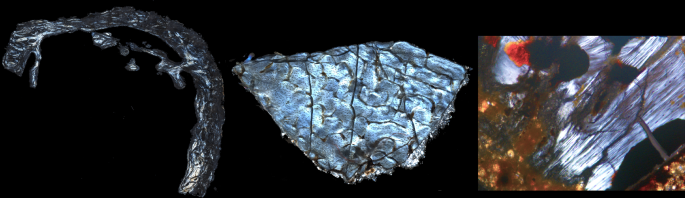
Palaeometabolomes yield biological and ecological profiles at early human sites
Zhang, A., Sun, H., Wang, P., Han, Y. & Wang, X. Recent and potential developments of biofluid analyses in metabolomics. J. Proteomics 75, 1079–1088 (2012).
Google Scholar
…Continue Reading
NASA Study Suggests Saturn’s Moon Titan May Not Have Global Ocean
A key discovery from NASA’s Cassini mission in 2008 was that Saturn’s largest moon Titan may have a vast water ocean below its hydrocarbon-rich surface. But reanalysis of mission data suggests a more complicated picture: Titan’s interior…
Continue Reading

Colliding galaxies create the brightest, fastest growing black holes at their centre
New data confirm that the titanic collisions of galaxies ignite the most powerful active galactic nuclei.
Active galactic nuclei (AGN) are phases in which supermassive black holes at the centre of galaxies actively feed on matter…
Continue Reading

NASA Study Suggests Saturn’s Moon Titan May Not Have Global Ocean
To remotely probe planets, moons, and asteroids, scientists study the radio frequency communications traveling back and forth between spacecraft and NASA’s Deep Space Network. It’s a multilayered process. Because a moon’s body may not have…
Continue Reading

A quantum mystery that stumped scientists for decades is solved
A global research team led by Rice University physicist Pengcheng Dai has verified the presence of emergent photons and fractionalized spin excitations in an unusual quantum spin liquid. Reported in Nature Physics, the work points to the crystal…
Continue Reading

From prey to predator: how carnivores spread beneficial fungi
Animals help disperse seeds and spores for many plant and fungal species. This typically happens when animals eat the fruiting bodies of plants and fungi and pass seeds and spores through their digestive systems.
Mycorrhizal…
Continue Reading

Backyard insect inspires large-scale invisibility particles production
BYLINE: Jamie Oberdick
UNIVERSITY PARK, Pa. — When most people see a leafhopper in their backyard garden, they notice little more than a tiny green or striped insect flicking…
Continue Reading