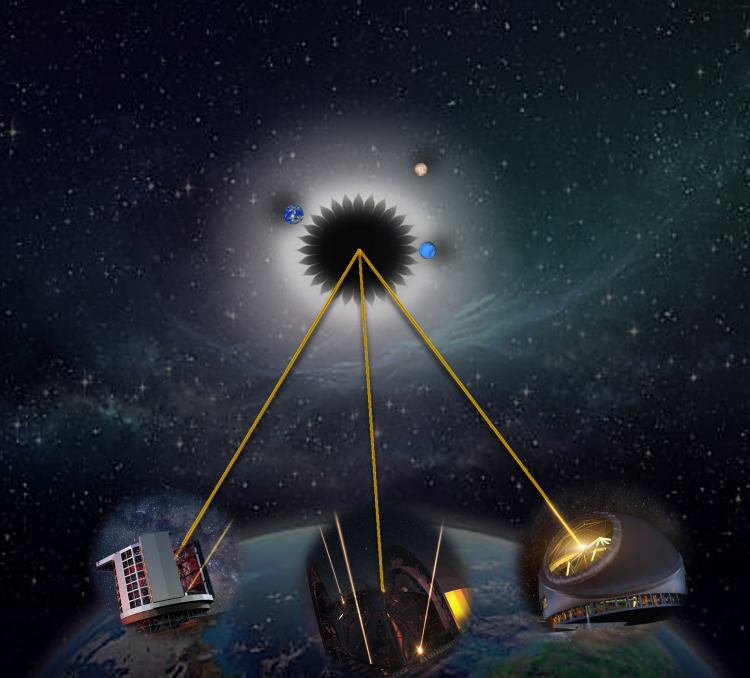
Finding Earth-like exoplanets with the composition and ingredients for life as we know it is the Holy Grail of exoplanet hunting. Since the first exoplanets were identified in the 1990s, scientists have pushed the boundaries of finding…
Category: 7. Science
-
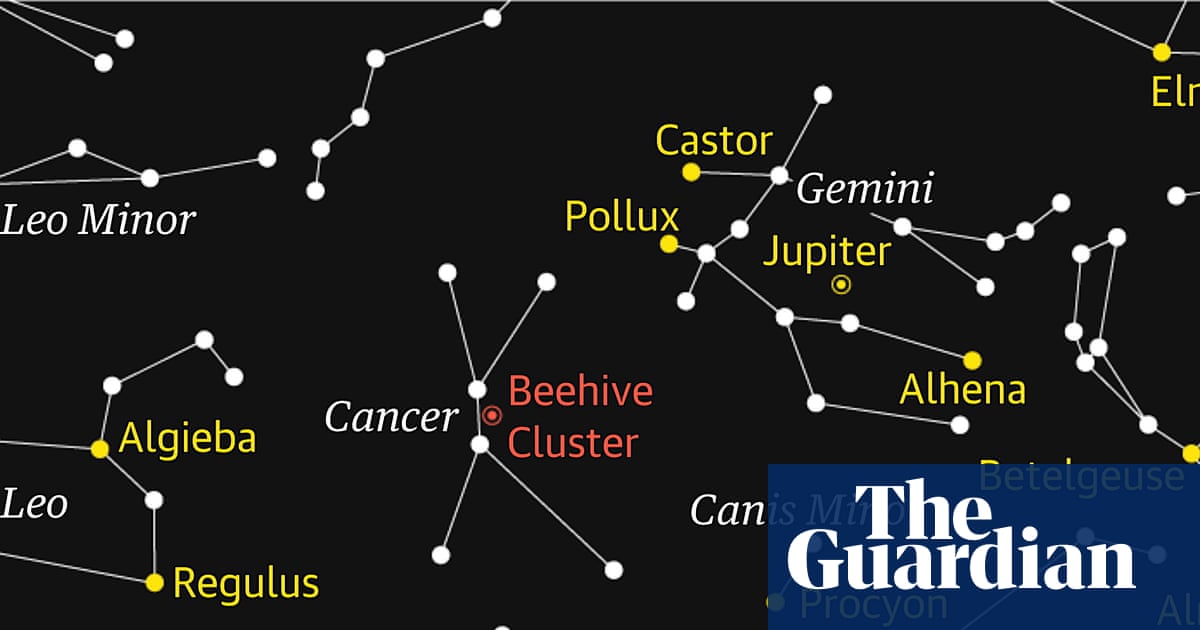
Starwatch: patience is needed to observe Cancer’s beehive cluster | Astronomy
The constellation of Cancer, the crab, is now high in the southern sky during the late evening. While not the easiest constellation to locate because it does not contain any truly bright stars, it does offer a reward for patient observation: the…
Continue Reading
-
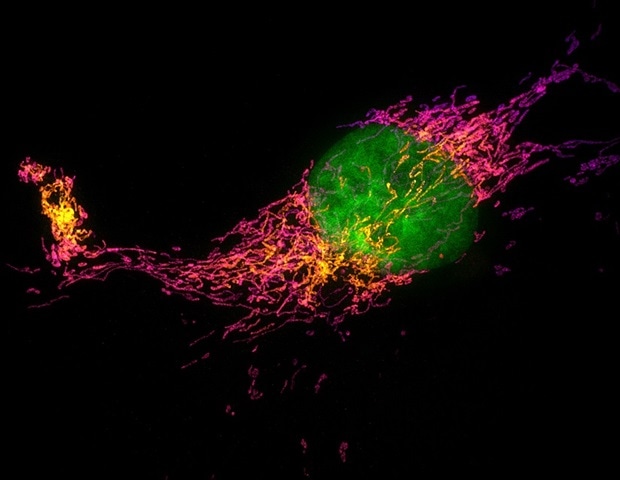
Understanding PIEZO2 mutations and sensory disorders
Every time we feel a gentle tap on the skin, specialized nerve cells convert that physical force into an electrical signal that the brain can interpret as touch. While scientists have long known that a protein called PIEZO2 acts as…
Continue Reading
-

Course Correction or Controlled Crash? Inside NASA’s Artemis Overhaul
by Clarence Oxford
Los Angeles CA (SPX) Mar 09, 2026NASA says it has finally found the “back to basics” recipe to get Americans back on the Moon by 2028. A new intermediate mission, standardized hardware, a faster launch cadence: on…
Continue Reading
-
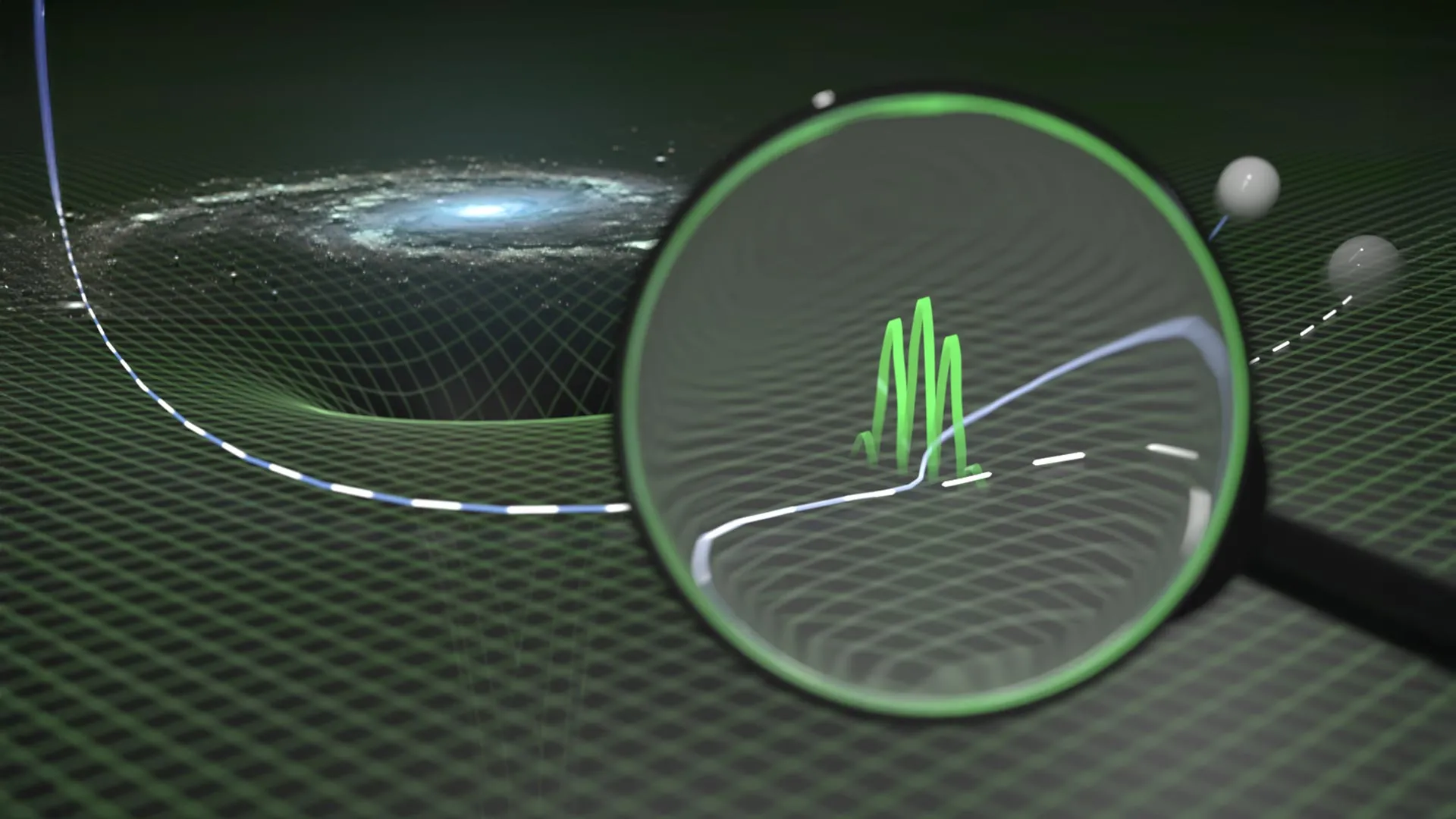
Particles may not follow Einstein’s paths after all
One of the biggest unsolved challenges in modern physics is bringing together two powerful theories that describe very different parts of reality. Quantum theory explains the behavior of extremely small particles with remarkable precision….
Continue Reading
-
“At First, We Thought Something Was Wrong” – NASA DART Mission Reveals a Cosmic Snowball Fight – SciTechDaily
- “At First, We Thought Something Was Wrong” – NASA DART Mission Reveals a Cosmic Snowball Fight SciTechDaily
- Moons around asteroids exchange dust and rocks, reveals study Interesting Engineering
- Dimorphos with color enhanced markings to…
Continue Reading
-

Study on rare earth formation sheds light on future discoveries
Recent research by Chinese scientists explains how deposits of rare earth elements (REE) are formed, proving that the emplacement depth (pressure) of carbonatitic magma is a key factor controlling the abnormal…
Continue Reading
-
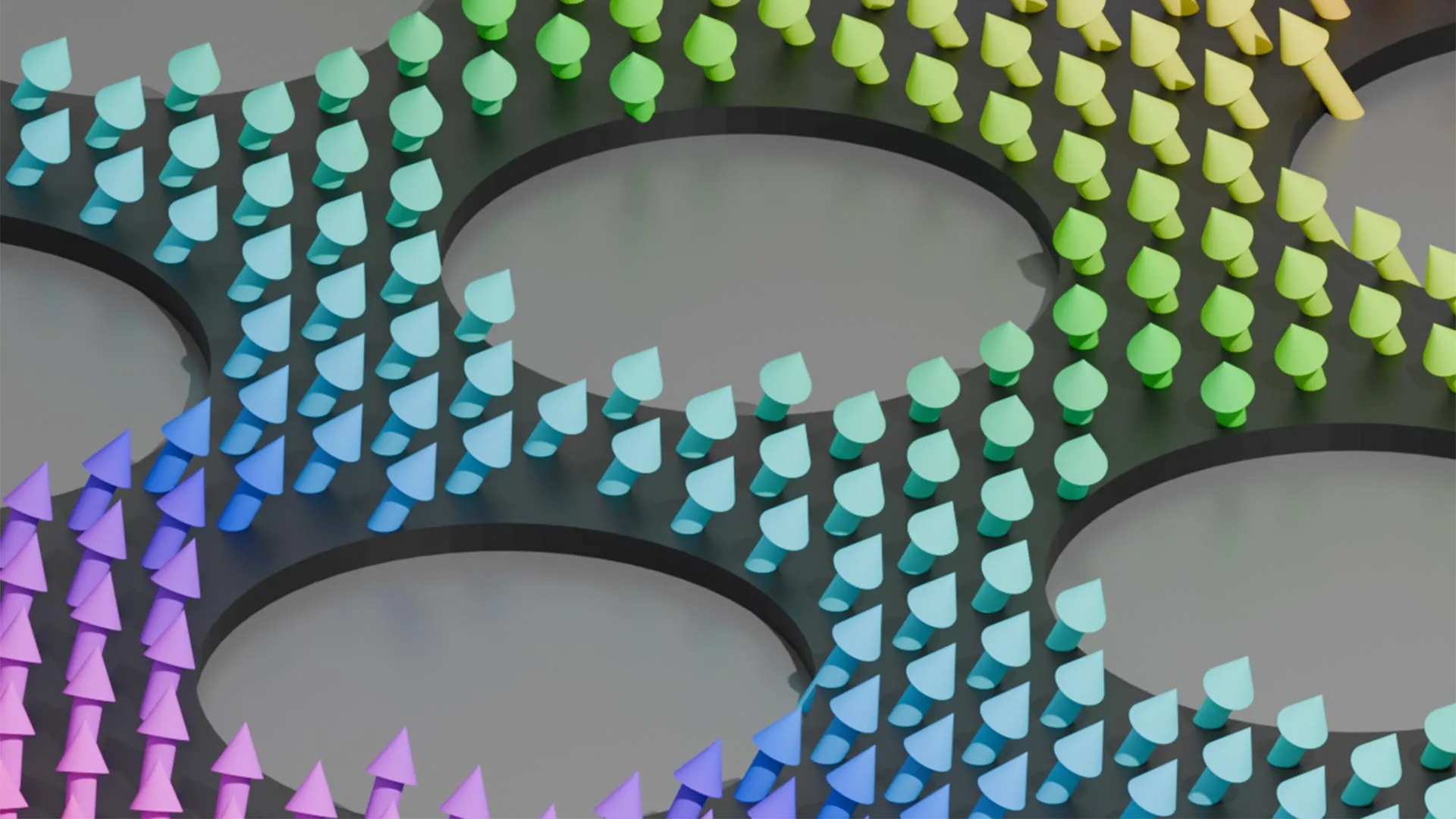
Engineers make magnets behave like graphene
Two dimensional materials have drawn intense interest because their electronic and magnetic properties could power future technologies. Scientists have traditionally treated these two behaviors as separate. Engineers at Illinois Grainger…
Continue Reading
-

Astronomers Produce the Largest Image Ever Taken of the Heart of the Milky Way
The central region of our Milky Way, sometimes referred to as the “Bulge,” remains something of an enigma to astronomers. Because it is densely packed with stars and clouds of dust and gas, capturing images of its interior has…
Continue Reading
-
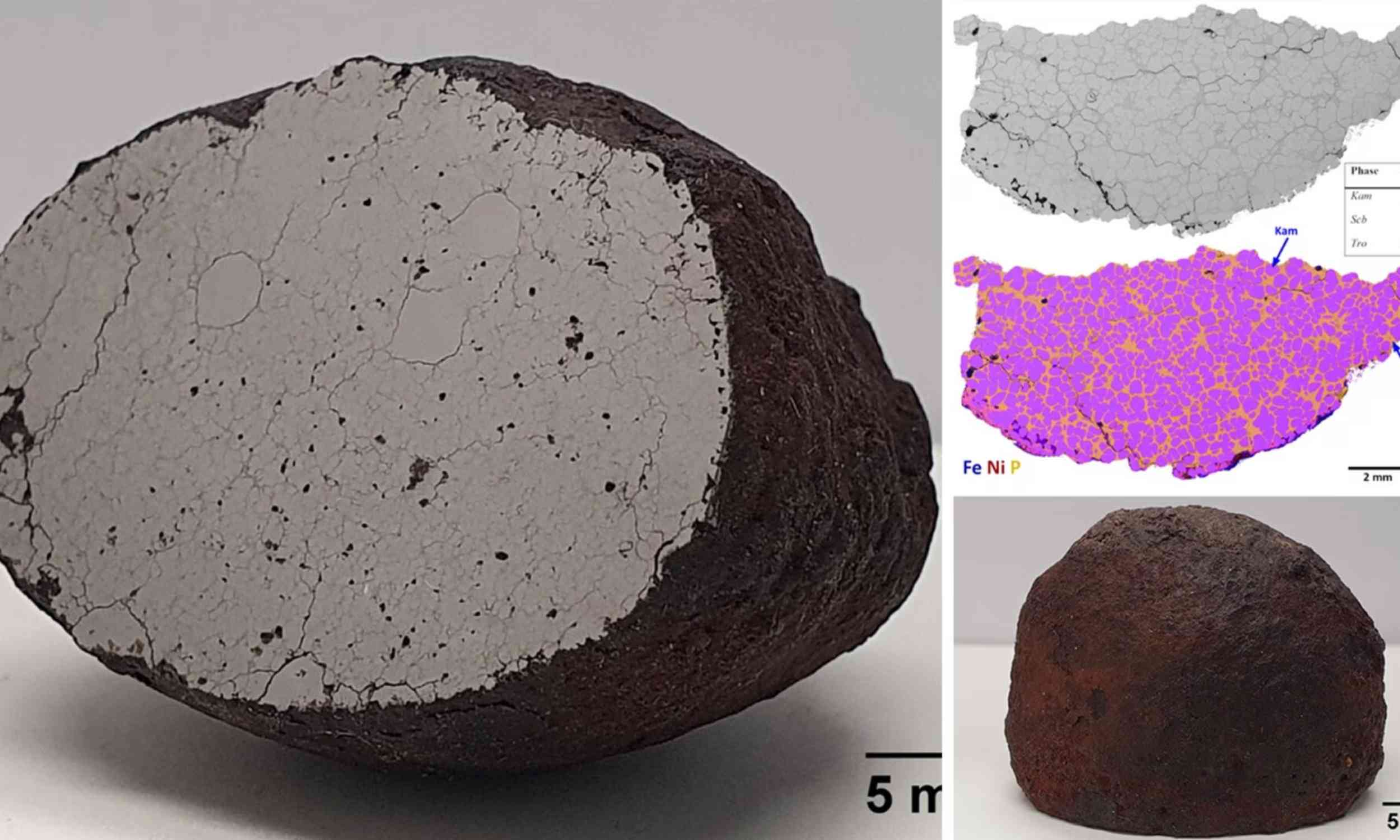
Space rock found in 2017 turns out to be incredibly rare
A small iron meteorite found in Finland has become the most phosphorus-rich iron meteorite ever identified.
Its extreme chemistry preserves a rare record of how metal once separated and reorganized inside a shattered asteroid core.
Inside the cut…
Continue Reading