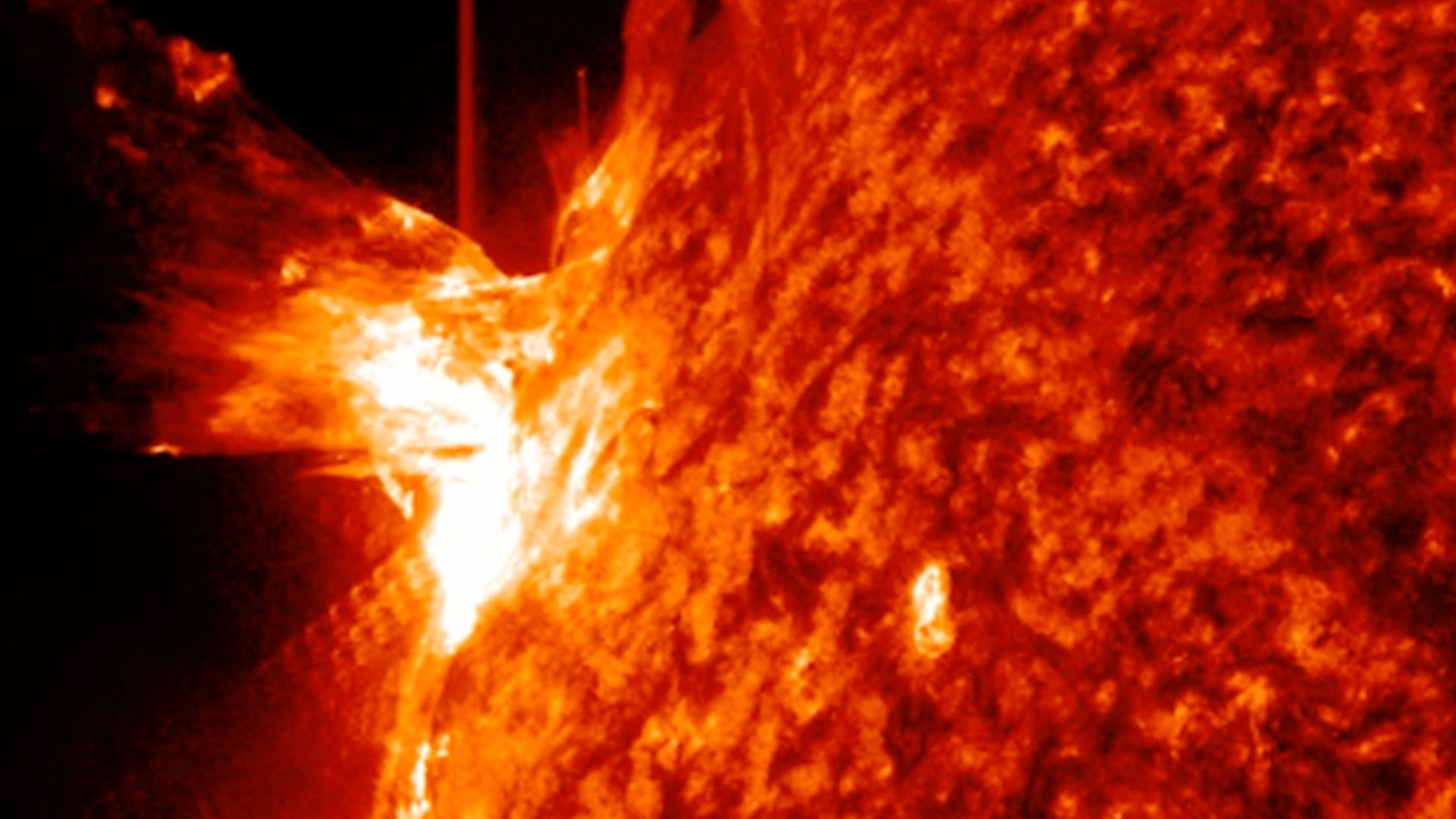
The sun kicked off December with a bang, unleashing a strong X1.9-class solar flare that briefly knocked out radio communications across Australia and parts of southeast Asia.
Category: 7. Science
-
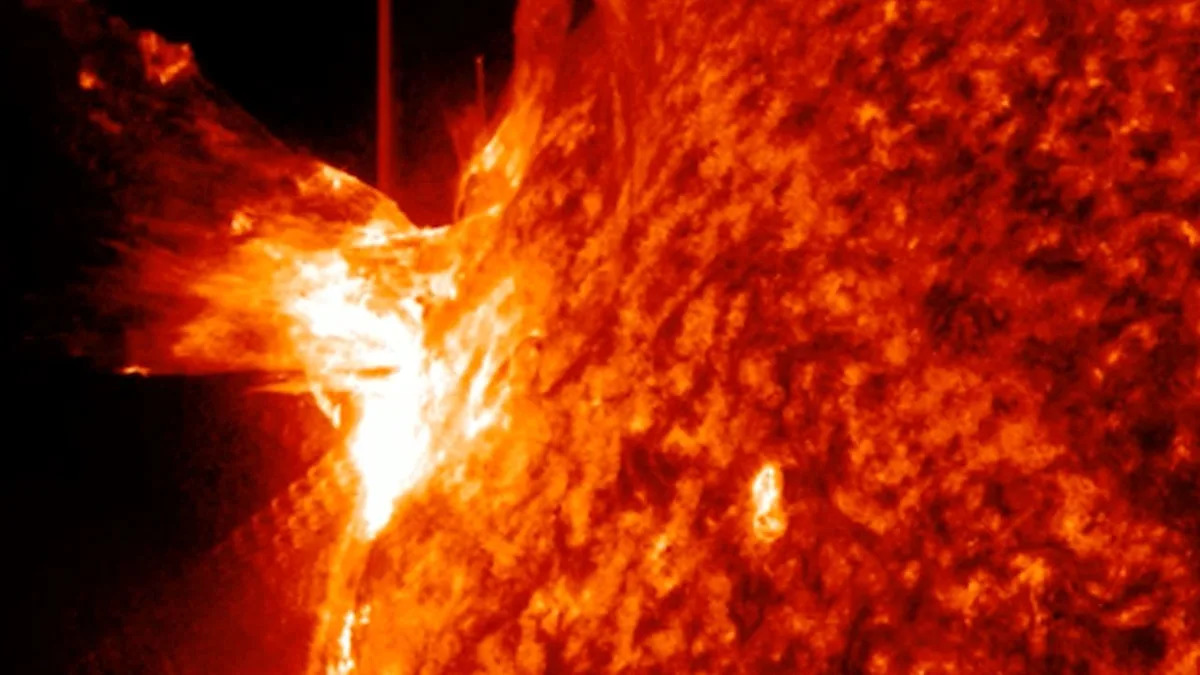
Sun unleashes powerful X-class solar flare, knocking out radio signals across Australia
When you buy through links on our articles, Future and its syndication partners may earn a commission.
A powerful X1.9 solar flare from new sunspot AR4299 triggered strong radio blackouts, as giant sunspot AR4294 rotates into view with more…
Continue Reading
-
All life copies DNA unambiguously into proteins. Archaea may be the exception.
The beauty of the DNA code is that organisms interpret it unambiguously. Each three-letter nucleotide sequence, or codon, in a gene codes for a unique amino acid that’s added to a chain of amino acids to make a protein.
But…
Continue Reading
-
Archaea May Break DNA-to-Protein Rule
The beauty of the DNA code is that organisms interpret it unambiguously. Each three-letter nucleotide sequence, or codon, in a gene codes for a unique amino acid that’s added to a chain of amino acids to make a protein.
But University of…
Continue Reading
-

Reef protections are slowing crown-of-thorns starfish outbreaks

One of the most extensive marine conservation initiatives ever undertaken on the Great Barrier Reef is starting to deliver some positive impact and helping to reduce the scale…
Continue Reading
-

Optics & Photonics News – Molecules Switch On Insulating Light Emitters
Lanthanide-doped nanostructures can be tailored to emit light at precise wavelengths across the visible spectrum and into the near infrared [Image: National University of Singapore]
Two independent research teams have devised similar…
Continue Reading
-

DNA transcription is a tightly choreographed event. A new study reveals how it is choreographed.
Life’s instructions are written in DNA, but it is the enzyme RNA polymerase II (Pol II) that reads the script, transcribing RNA in eukaryotic cells and eventually giving rise to proteins. Scientists know that Pol II must advance down the gene…
Continue Reading