Physicists have checked again whether light of different colors travels at different speeds, and once more it seems not to budge.
They timed energetic photons from distant cosmic blasts to see whether high energy ones ever reached their detectors…

Physicists have checked again whether light of different colors travels at different speeds, and once more it seems not to budge.
They timed energetic photons from distant cosmic blasts to see whether high energy ones ever reached their detectors…
Nasa has confirmed it will continue supporting the European Space Agency’s Rosalind Franklin Mars rover, which is now slated for a 2028 launch after a series of setbacks. The ESA announced the update on Wednesday during its ministerial…

On the morning of June 30, 1908, residents scattered across the Siberian taiga awoke to a sky that seemed to split in two — an episode now inseparable from discussions of planetary risk and, increasingly, tools like ΕΝΕΟ that help interpret…

Coastlines around the planet are being steadily “crushed” as climate-driven sea level rise combines with expanding development in coastal zones. This ongoing process damages the diverse life that depends on sandy environments, disrupts local…
LONDON, Nov. 27 (Xinhua) — Researchers have detected electrical discharges dubbed “micro-lightning” in the Martian atmosphere for the first time, according to a study published Wednesday in the journal Nature.
As well as on Earth, lightning…

If you’re cooking a curry, Ashish Kumar has a piece of advice – switch on your extractor fan. Or, if that’s not an option, open a window. Why? Because his research shows that cooking curries in poorly ventilated spaces can lead to high…

Black holes keep showing up in the news. Scientists have recorded the space ripples from their violent crashes and even captured the first images of their shadowed outlines at the centers of galaxies. These breakthroughs have driven fresh…
![Best Space Shots of the Week: From Ground-Based Gazers to Spacefaring Eyes [20-27 Nov]](https://afnnews.qaasid.com/wp-content/uploads/2025/11/best-space-shots.jpg)
This week’s best space shots collection offers a rare blend of perspectives from across the cosmos, from backyard telescopes to the windows of Earth orbit. Amateur astrophotographers capture nebulae, planets, and lunar scenes with astonishing…
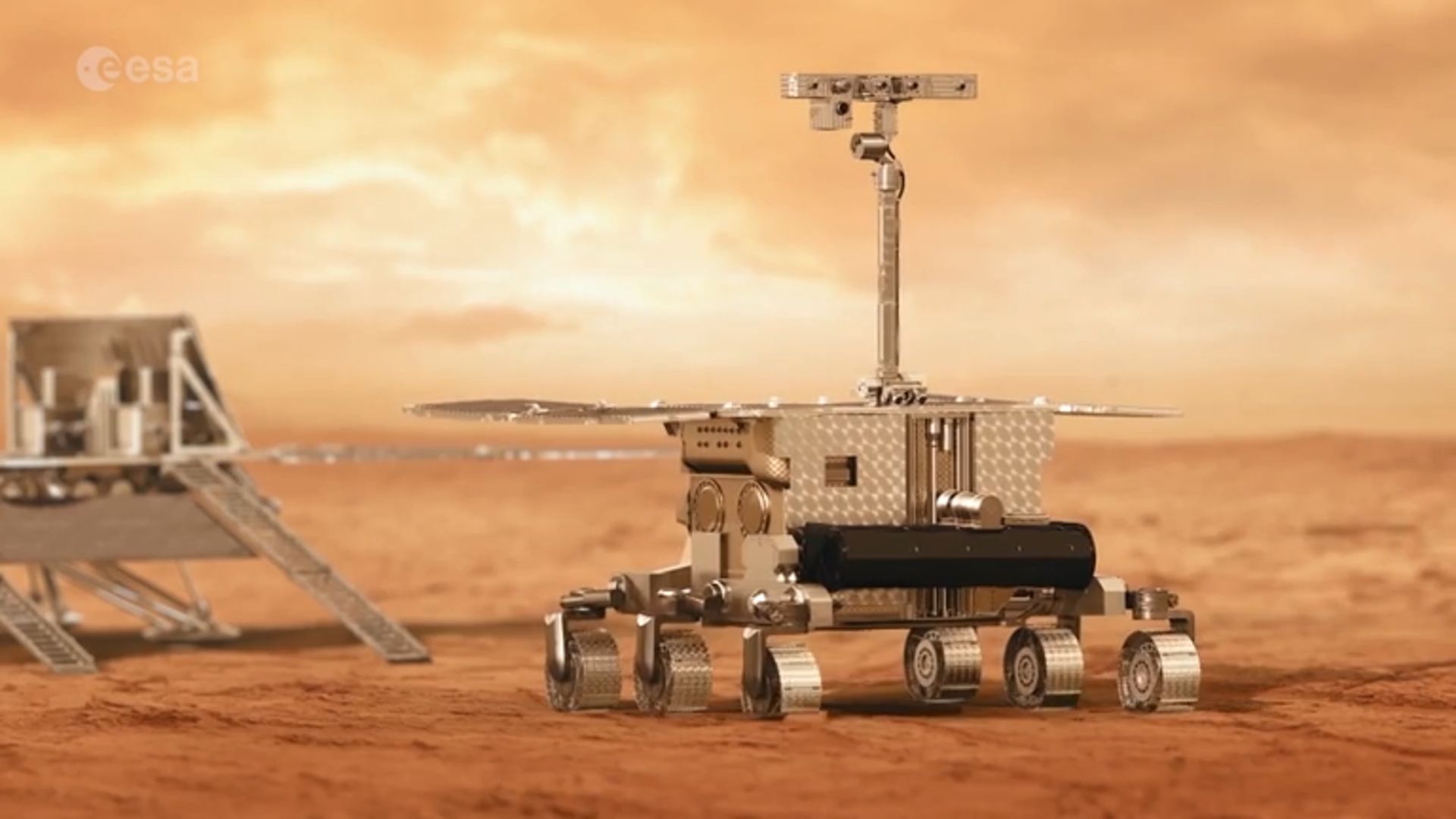
BREMEN, Germany — NASA will help Europe get its long-delayed Mars life-hunting ExoMars rover off the ground, even though President Donald Trump’s proposed NASA budget cuts the collaboration in a drive to reduce spending on science.
The…

Are you ready for a year of stargazing? Skywatchers have a plethora of fascinating sights in late-2025 and throughout 2026, chief among them a couple of eclipses — a ‘blood moon’ total lunar eclipse on March 3, 2026, followed by a total…