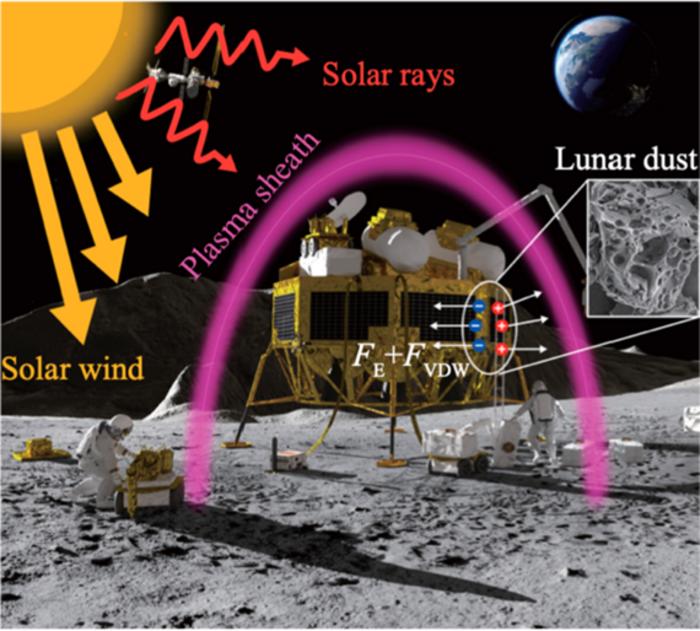
Understanding how exactly lunar dust sticks to surfaces is going to be important once we start having a long-term sustainable presence on the Moon. Dust on the Moon is notoriously sticky and damaging to equipment, as well as being…
We’re on day five of the lunar cycle, which means the moon is working on getting bigger every night. There will be more coming into view tonight than last, so keep reading to find out…