Astronomers have just logged the 40,000th near-Earth asteroid, a major marker in humanity’s effort to keep track of the rocks that pass close to our planet.
These objects range from a few yards across to a couple of miles wide. Every new one…
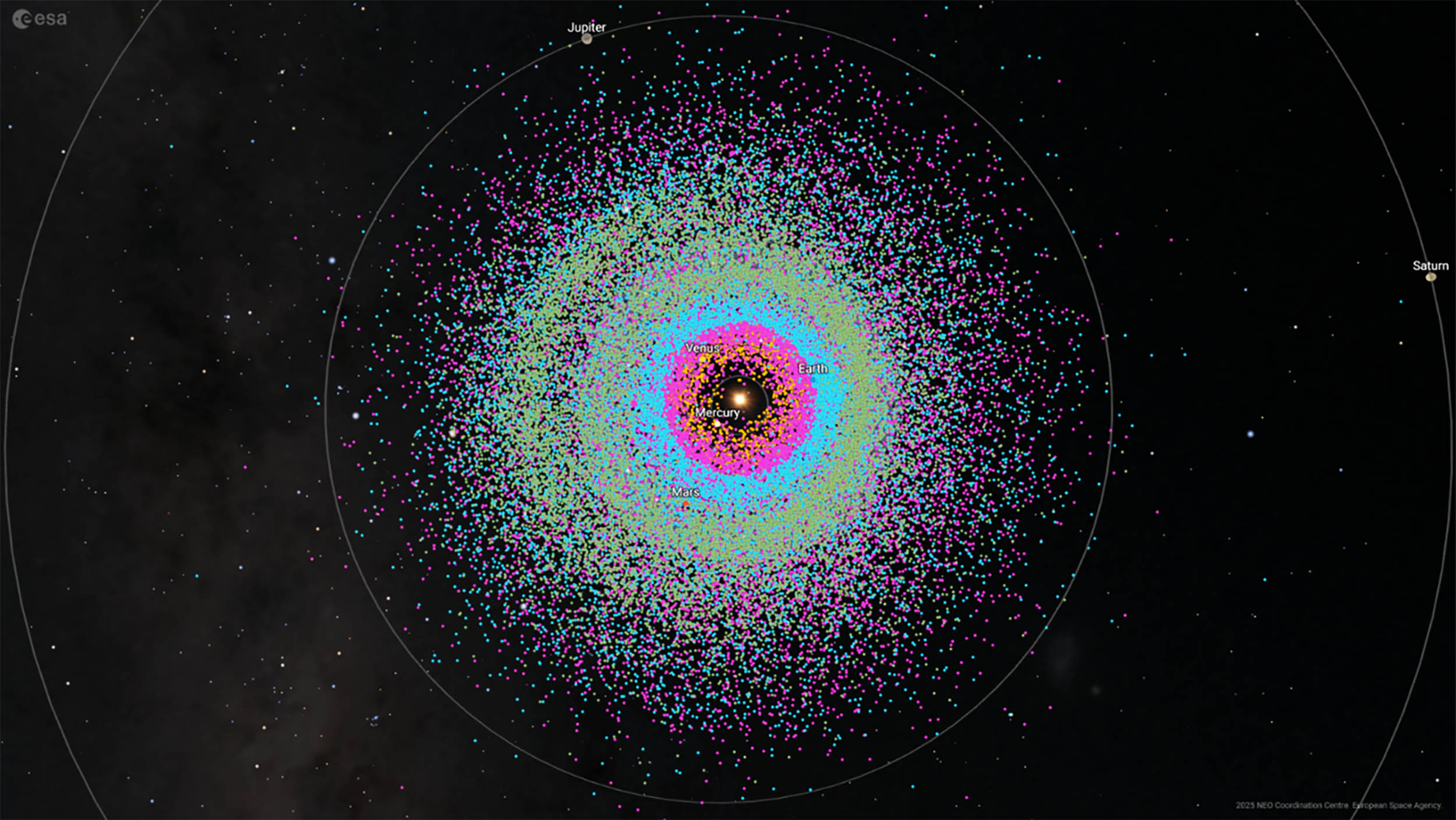
Astronomers have just logged the 40,000th near-Earth asteroid, a major marker in humanity’s effort to keep track of the rocks that pass close to our planet.
These objects range from a few yards across to a couple of miles wide. Every new one…

Eric Boyd and his research team investigated how a burst of small earthquakes in 2021 affected the communities of microbes living deep beneath the Yellowstone Plateau Volcanic Field. These microbes inhabit rock and water systems far below the…

The rapid retreat of glaciers, an increasingly common phenomenon, could potentially lead to complex changes in ocean chemistry. A new study has revealed that sediment runoff from retreating glaciers is less nutritious for marine life than…

One fateful day about 4.5 billion years ago, a Mars-sized body called Theia collided with proto-Earth, turning both into a molten mess of rock and metal. Once the debris had coalesced, two distinct objects remained locked in an orbit –…

The quest to grow food beyond Earth has fascinated scientists and space agencies for decades, not only as a matter of survival,…

Starliner is working on its redemption arc.
After making headlines for a troubled crew flight test that ended last year, as well as two uncrewed flight tests in 2019 and 2022 that did not meet expectations, the Boeing capsule is trying to shed…
There are few forms of the botanical world as readily identifiable as fern leaves. These often large, lacy fronds lend themselves nicely to watercolor paintings and tricep tattoos alike. Thoreau said it best: “Nature made ferns for pure…

When polar ice sheets melt, the effects ripple across the world. The melting ice raises average global sea level, alters ocean currents and affects temperatures in places far from the poles.
But melting ice sheets don’t affect sea level…

For Chilean astrophotographer Osvaldo Castillo, the night sky above the European Southern Observatory‘s (ESO) Paranal Observatory held a breathtaking sight.
“I couldn’t believe I was photographing a circumpolar startrail in Paranal; without a…