Small differences in a newborn’s fingers may carry surprising clues about early brain growth. A new study finds that boys with certain finger-length patterns tend to be born with larger head circumferences.
This pattern suggests that hormone…

Small differences in a newborn’s fingers may carry surprising clues about early brain growth. A new study finds that boys with certain finger-length patterns tend to be born with larger head circumferences.
This pattern suggests that hormone…
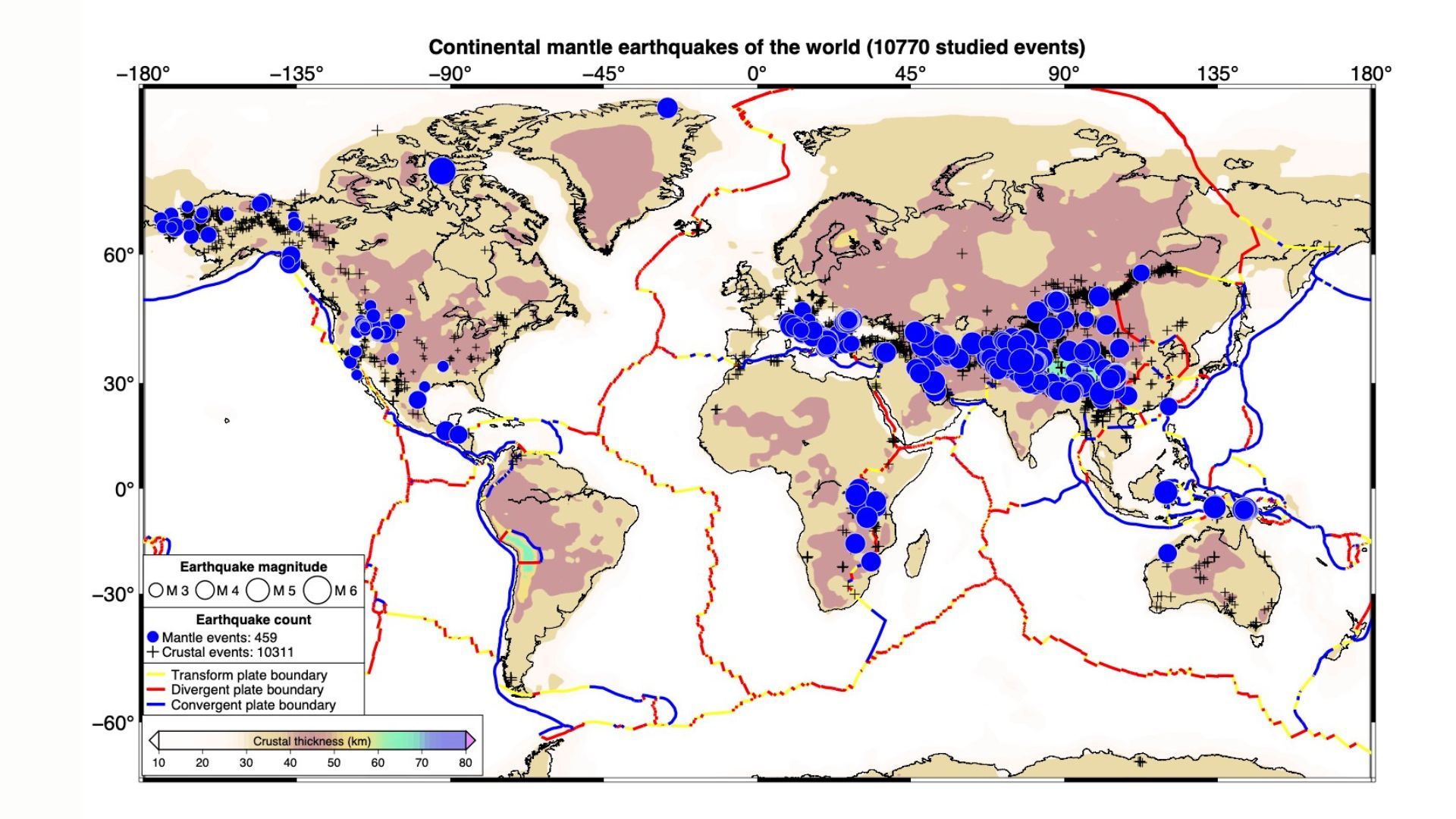
Earthquakes that jiggle Earth’s middle layer may be more widespread than scientists thought.
A new map of these mysterious deep earthquakes shows that they occur all around the world and that they may have a variety of causes. That’s…

A new analysis suggests parts of Earth’s climate system may be approaching dangerous tipping points sooner than scientists once expected.
The concern is that once certain thresholds are crossed, powerful feedback loops could accelerate warming…

Last year, NASA’s Curiosity rover made a fascinating discovery after boring into a suspected ancient lake bed on Mars: long-chain organic molecules, called alkanes, that could serve as a potential chemical relic of ancient life on the Red…

Refresh
Good morning, Space Fans! Welcome to docking day for NASA’s Crew-12 astronauts, and it will be a match made in the stars as the SpaceX Dragon…
This week in science: Mysterious molecules on Mars are tricky to explain without life; a compound that cuts cholesterol as a daily oral pill; an experimental new treatment for sleep apnea has a 93 percent success rate; and much more!

Watch On
Space fans are in for a Valentine’s Day treat.
Brito, P. M., Meunier, F., Clément, G. & Geffard-Kuriyama, D. The histological structure of the calcified lung of the fossil coelacanth Axelrodichthys araripensis (Actinistia: Mawsoniidae). Palaeontology 53, 1281–1290 (2010).

“Ghost lineages” may sound paranormal, but the term is rooted in real science that genetic studies have revealed only relatively recently.
So what is a ghost lineage?

Spectroscopy published an online content series, titled “Unsolved Problems in Spectroscopy,” which explored evolving intersection of advanced computational methods and spectroscopic analysis, focusing on the limitations of traditional linear…