Experiments using conditional knockout mice have revealed a critical role for stress signaling-mediated transcriptional responses in determining the fates of neurons after axon injury. Prominent among these, neuronal knockout of the…
Category: 7. Science
-
Chinese scientists unveil AI model to unify stellar data from global telescopes – TV BRICS
- Chinese scientists unveil AI model to unify stellar data from global telescopes TV BRICS
- Chinese researchers develop AI model to process stellar data from different telescopes news.cgtn.com
- Chinese scientists unveil advanced AI model to support…
Continue Reading
-

James Webb Space Telescope performs brain surgery on mysterious ‘Exposed Cranium Nebula’
The James Webb Space Telescope’s latest imagery is its most “cerebral” yet, capturing a dying star’s nebula that looks uncannily like a brain inside a transparent skull.
Located about 5,000 light-years away in the constellation of Vela, the…
Continue Reading
-
Terrifying New Details Emerge on Last Year's Stranding of Chinese Taikonauts – extremetech.com
- Terrifying New Details Emerge on Last Year’s Stranding of Chinese Taikonauts extremetech.com
- ‘Some of the cracks had penetrated through’: Chinese astronauts reveal new details about spacecraft that ‘stranded’ them in space last year Space
- Damage…
Continue Reading
-
Ancient lizard with bone cancer found preserved in amber: study-Xinhua
BEIJING, Feb. 26 (Xinhua) — A team of paleontologists has discovered the first-known evidence of a bone tumor in a vertebrate preserved in amber, in the form of a 99-million-year-old lizard specimen that offers a rare glimpse into the ancient…
Continue Reading
-

Multiplex biomarker platforms in neurodegenerative diseases
With few biomarkers for early identification and limited therapy options for conditions such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), there is an urgent need for further information about the biology of disease genesis and…
Continue Reading
-

9 night sky events to see in March, from a blood moon lunar eclipse to a planetary parade
Total lunar eclipse—March 3
All eyes will point skyward in the early morning hours of March 3. The sight—a total lunar eclipse—will turn the full worm moon a haunting tangerine hue. The mechanics of a total lunar eclipse trigger this blood…
Continue Reading
-
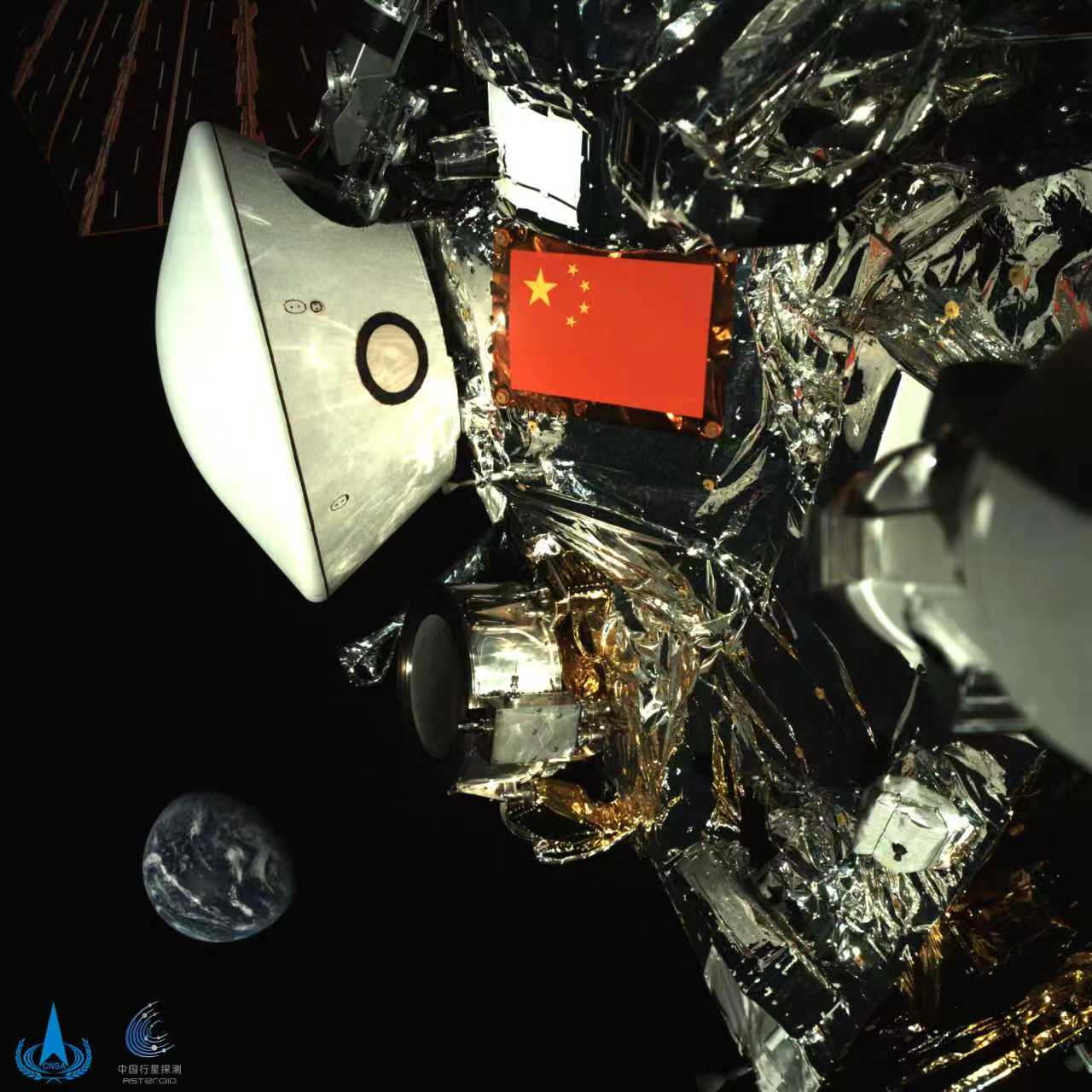
China’s Tianwen-2 probe operating normally on approach to asteroid
HELSINKI — China’s Tianwen-2 spacecraft is operating normally on its way to a near-Earth asteroid ahead of sampling later this year, according to a rare official update.
Tianwen-2 launched May 28 last year, embarking on a voyage to…
Continue Reading
-
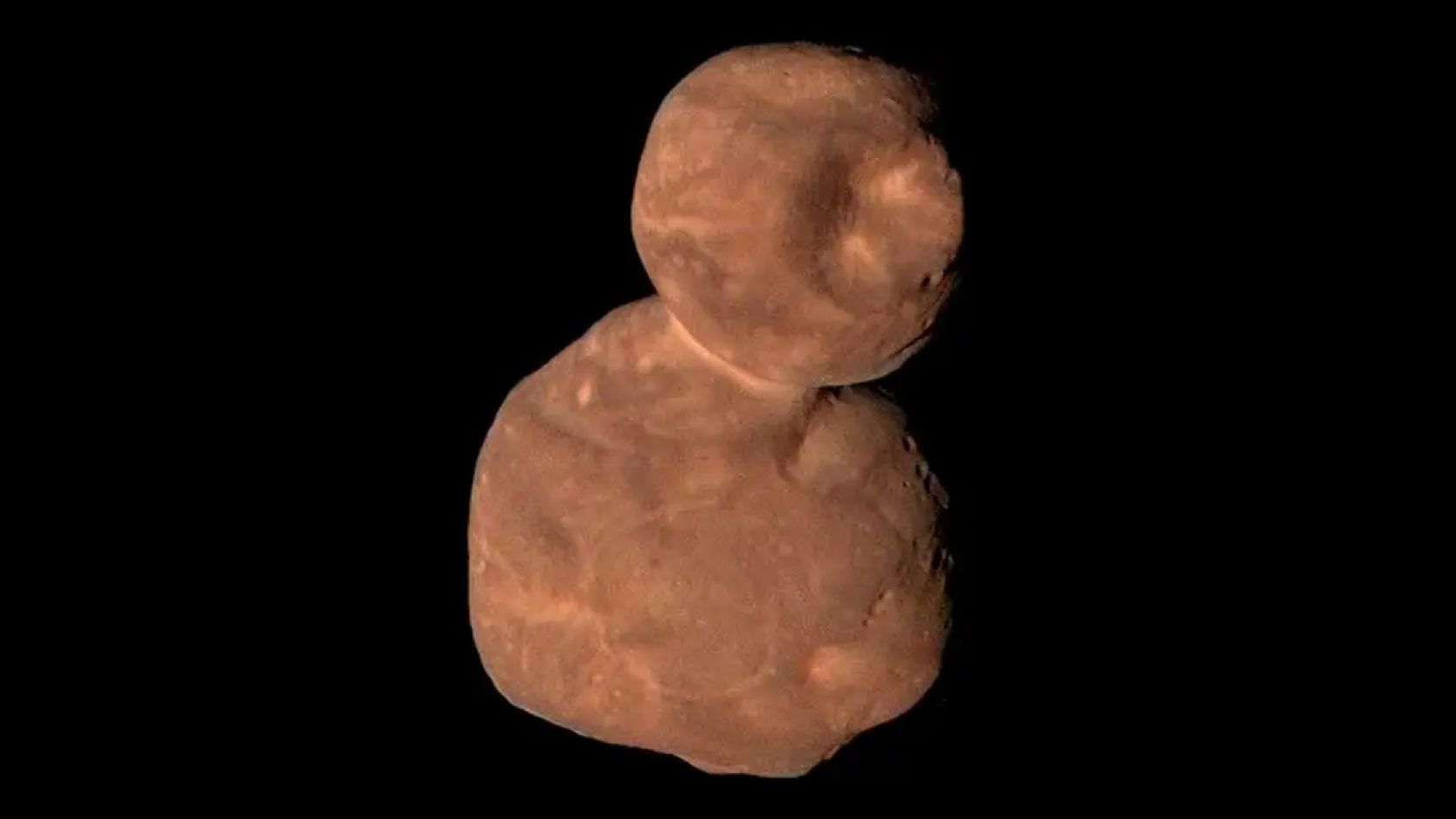
Why are there so many ‘space snowmen’ in our solar system? New study offers clues
In the distant reaches of the solar system are many icy objects that resemble snowmen — pairs of conjoined spheres. Now, a new study reveals the simple way in which these mysterious objects might form.
Beyond the orbit of Neptune lie icy…
Continue Reading
-

Sensors Information | AZoSensors.com – Page not found
Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…Continue Reading
