Across much of human history, women and children have kept camps alive by transforming bitter, inedible plants into safe, calorie-rich meals when hunting failed.
A new analysis argues that this daily work of processing and cooking food helped…

Across much of human history, women and children have kept camps alive by transforming bitter, inedible plants into safe, calorie-rich meals when hunting failed.
A new analysis argues that this daily work of processing and cooking food helped…

When AI powered prosthetic arms that move autonomously become widespread, understanding how people feel about them and accept them will be crucial. In this study, we used virtual reality to simulate a situation in which a…

Scientists are increasingly focused on understanding how interventions impact individuals differently, yet disentangling these heterogeneous treatment effects remains difficult, particularly with complex, continuous data. Filippo Salmaso from…

Between 73,000 and 20,000 years ago (Late Pleistocene), the Japanese Archipelago was inhabited by cave lions (Panthera spelaea), according to a new genetic and proteomic analysis of fossilized felid remains previously attributed to tigers…
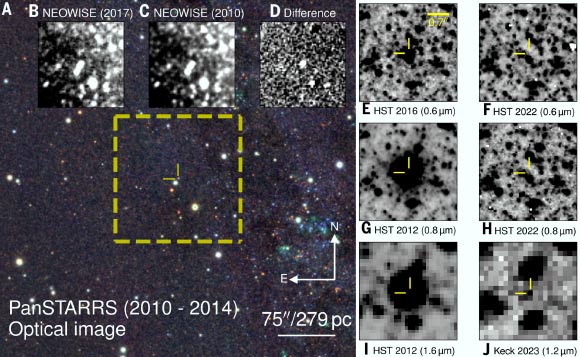
Using archival data from NASA’s NEOWISE mission along with data from other space and ground-based observatories, astronomers identified the clearest observational record yet of a massive star fading and vanishing into a black hole — an…

The world’s largest neutrino detector just got even more powerful after scientists successfully deployed six new strings of advanced sensors nearly 8,000 feet (2,400 meters) beneath the Antarctic ice at the IceCube Neutrino Observatory.
By…

Researchers are now challenging fundamental assumptions about the nature of gravity and its relationship to quantum statistics. Bekir Baytaş from Izmir Institute of Technology, Patrick Rodrigues and Nelson Yokomizo from Universidade Federal…
Researchers are developing a novel framework to enhance the precision of spatial quantum sensing, a crucial capability for diverse applications from large-scale geophysical surveys to localised biological measurements. Luís Bugalho and Yasser…
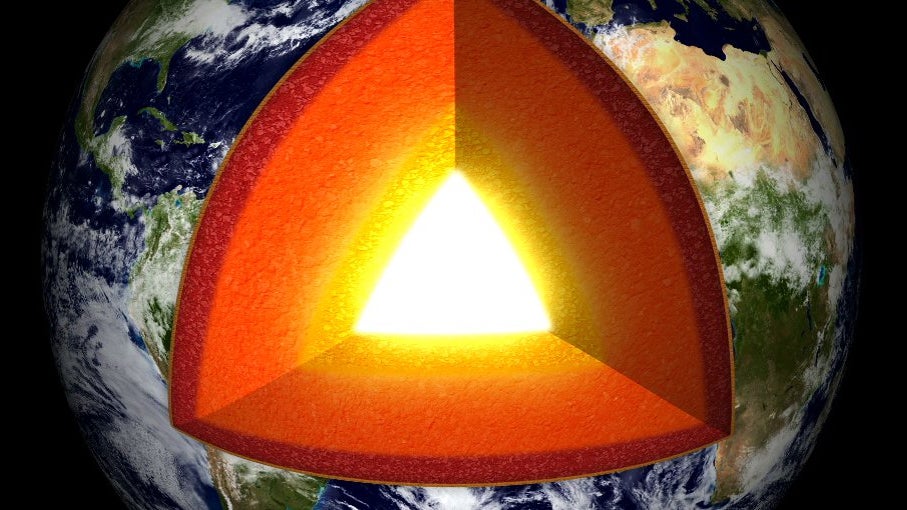
While we have sent probes billions of kilometers into interstellar space, humans have barely scratched the surface of our own planet, not even making it through the thin crust.
Information about Earth’s deep interior comes mainly from geophysics…