Outer space is having a moment. NASA’s Artemis 2 mission is about to take humans farther into space than we have ever gone; SpaceX is preparing to test the latest version of Starship, its interplanetary transport system; and just…
Category: 7. Science
-
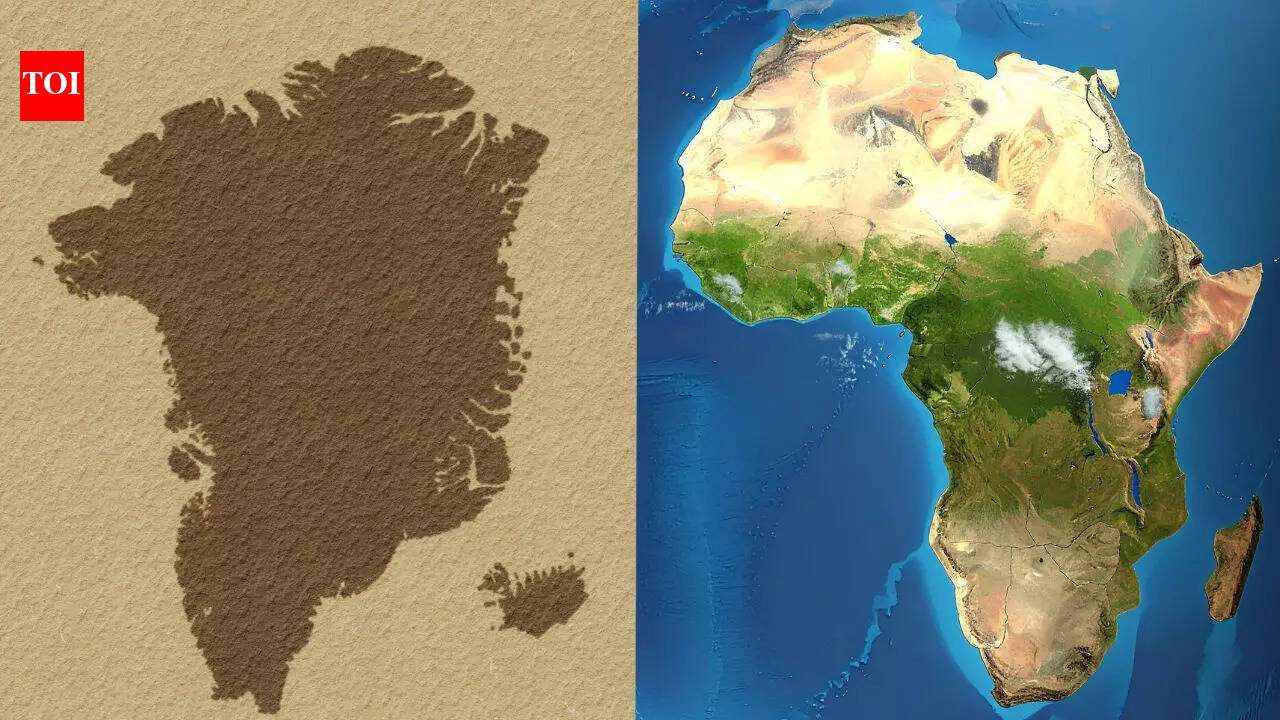
Why does Greenland appear bigger on maps than Africa, even though it is not | World News
Maps sit quietly in classrooms, on news websites and inside phone screens. They look settled and precise, but the world they show is always slightly bent out of shape….
Continue Reading
-

Ice Age fossils reveal how modern wolves are adapting to climate change
Science newsBy James Ashworth
Ancient wolf bones are revealing how today’s wolves might survive in a warmer world.
Higher temperatures in the past caused wolves to eat harder foods, suggesting major changes for…
Continue Reading
-

New Electrified Method Captures Carbon Dioxide from Air | News
Sargent is the Lynn Hopton Davis and Greg Davis Professor of Chemistry at the Weinberg College of Arts and Sciences, professor of electrical and computer engineering at Northwestern Engineering, and director of the Paula M. Trienens Institute for…
Continue Reading
-
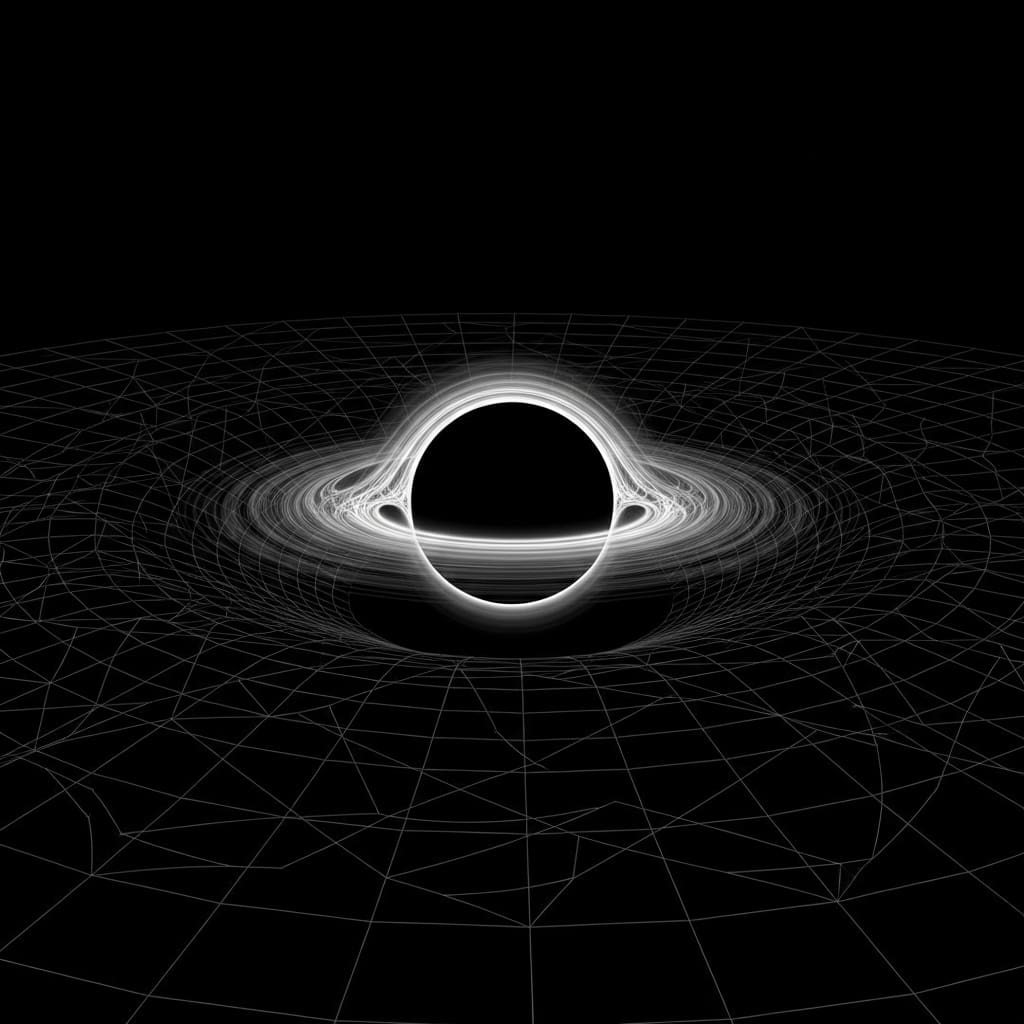
Ripples From Black Holes Could Reveal Flaws In Einstein’s Theory Of Gravity
Scientists are employing gravitational wave astronomy to probe the strong-field regime of gravity and test the predictions of general relativity. Dan Zhang, Chao Zhang, and Qiyuan Pan, alongside Guoyang Fu, Jian-Pin Wu et al., have…
Continue Reading
-

Scientists create glowing wax moths to study infections
Drug-resistant infections are becoming harder to treat, but testing new medicines in animals is often slow and expensive.
Scientists have now taken a major step forward by creating the first genetically modified wax moths – tiny larvae whose…
Continue Reading
-
Over 40% of Men Start Losing Their Y Chromosome By Age 60 and It Puts Their Hearts at Risk
Illustration by ZME Science. Men tend to lose the Y chromosome from their cells as they age. But because the Y bears few genes other than for male determination, it was thought this loss would not affect health.
But evidence has…
Continue Reading